Method for preparing aldehyde by alkene hydroformylation
An alkene hydroformyl and alkene technology, which is applied to the preparation of carbon monoxide reaction, chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problems of catalyst loss, high cost, and difficulty in realizing industrialization. , to achieve the effect of easy separation, avoid deactivation and decomposition, and excellently promote catalytic acceleration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
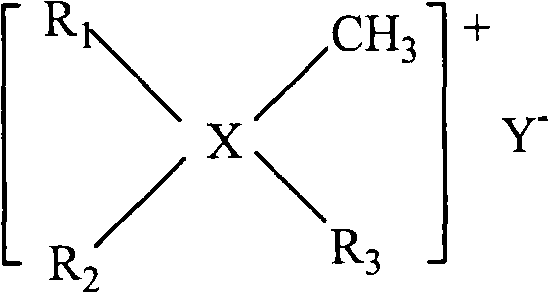
[0024] Example 1, the rhodium complex [RhCl (COD)] 2 1 part, phosphine ligand TPPTS 20 parts, double long-chain surfactant [N(CH 3 ) 2 (C 8 h 17 ) 2 ] 0.01 part of Cl, 500 parts of deionized water were added into a reactor with a stirrer to completely dissolve it, and the reactor was replaced 5 times with synthesis gas (hydrogen: carbon monoxide = 1:1). Add 100 parts of 1-butene, then add synthesis gas pressure to 2MPa, keep the partial pressure of synthesis gas constant, react at a temperature of 110°C for 6 hours, take out the reactant after cooling, and analyze the product content by gas chromatography: butene The conversion rate is 90%, the selectivity of forming aldehydes is greater than 98%, and the ratio of normal aldehydes to isomeric aldehydes is 4.1.
example 2
[0025] Example 2, the rhodium complex [RhCl (COD)] 2 1 part, phosphine ligand TPPDS 30 parts, double long-chain surfactant [N(CH 3 )(C 4 h 9 )(C 10 h 21 ) 2 ] 0.02 parts of Cl and 1000 parts of deionized water were added into a reactor equipped with a stirrer to completely dissolve it, and the reactor was replaced 3 times with synthesis gas (hydrogen: carbon monoxide = 1:1). Add 200 parts of 1-butene, and then add synthesis gas to a pressure of 2 MPa. Keep the partial pressure of synthesis gas constant during the reaction. React for 6 hours at a temperature of 100°C. After cooling, take out the reactant and analyze the product content by gas chromatography: The conversion rate of butene is 92%, the selectivity of forming aldehyde is greater than 98%, and the ratio of normal aldehyde and isomeric aldehyde is 4.5.
example 3
[0026] Example 3, the rhodium complex [RhCl (COD)] 2 2 parts, phosphine ligand TPPMS 30 parts, double long-chain surfactant [P(CH 3 ) 2 (C 10 h 21 ) 2 ] 0.04 parts of Cl and 1000 parts of deionized water were added into a reactor with a stirrer to completely dissolve it, and the reactor was replaced 3 times with synthesis gas (hydrogen: carbon monoxide = 1:1). Add 100 parts of 1-butene, then add synthesis gas pressure to 2MPa, keep the partial pressure of synthesis gas constant, react at a temperature of 100°C for 6 hours, take out the reactant after cooling, and analyze the product content by gas chromatography: butene The conversion rate is 93%, the selectivity of forming aldehydes is greater than 98%, and the ratio of normal aldehydes to isomeric aldehydes is 5.5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com