A kind of synthetic method of lamivudine intermediate
A synthesis method and cytosine technology, applied in the field of synthesis of lamivudine intermediates, can solve problems affecting the health and safety of operators, polluting the environment, and high toxicity of raw materials, achieving low production costs, simplified reaction steps, The effect of saving production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
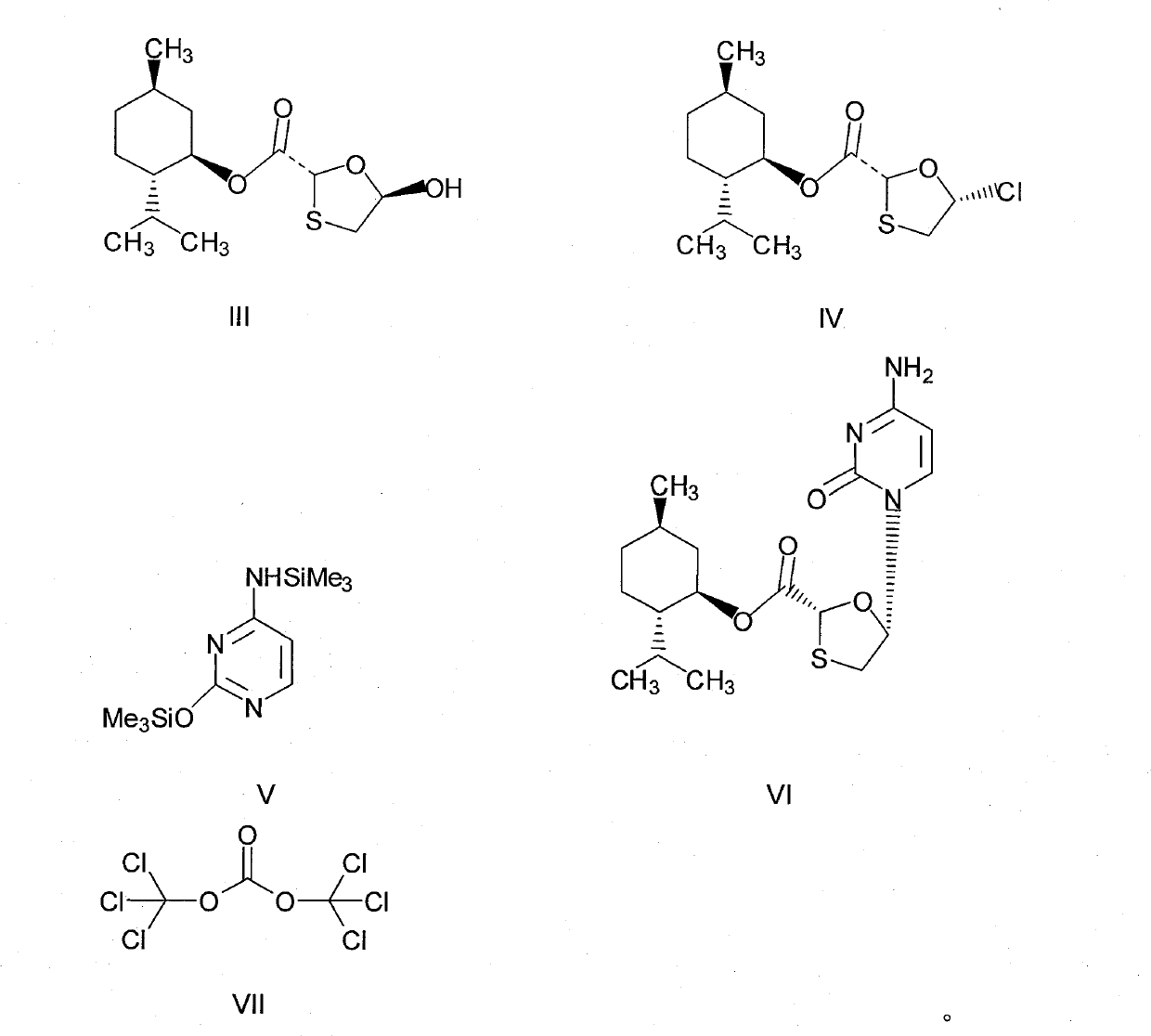
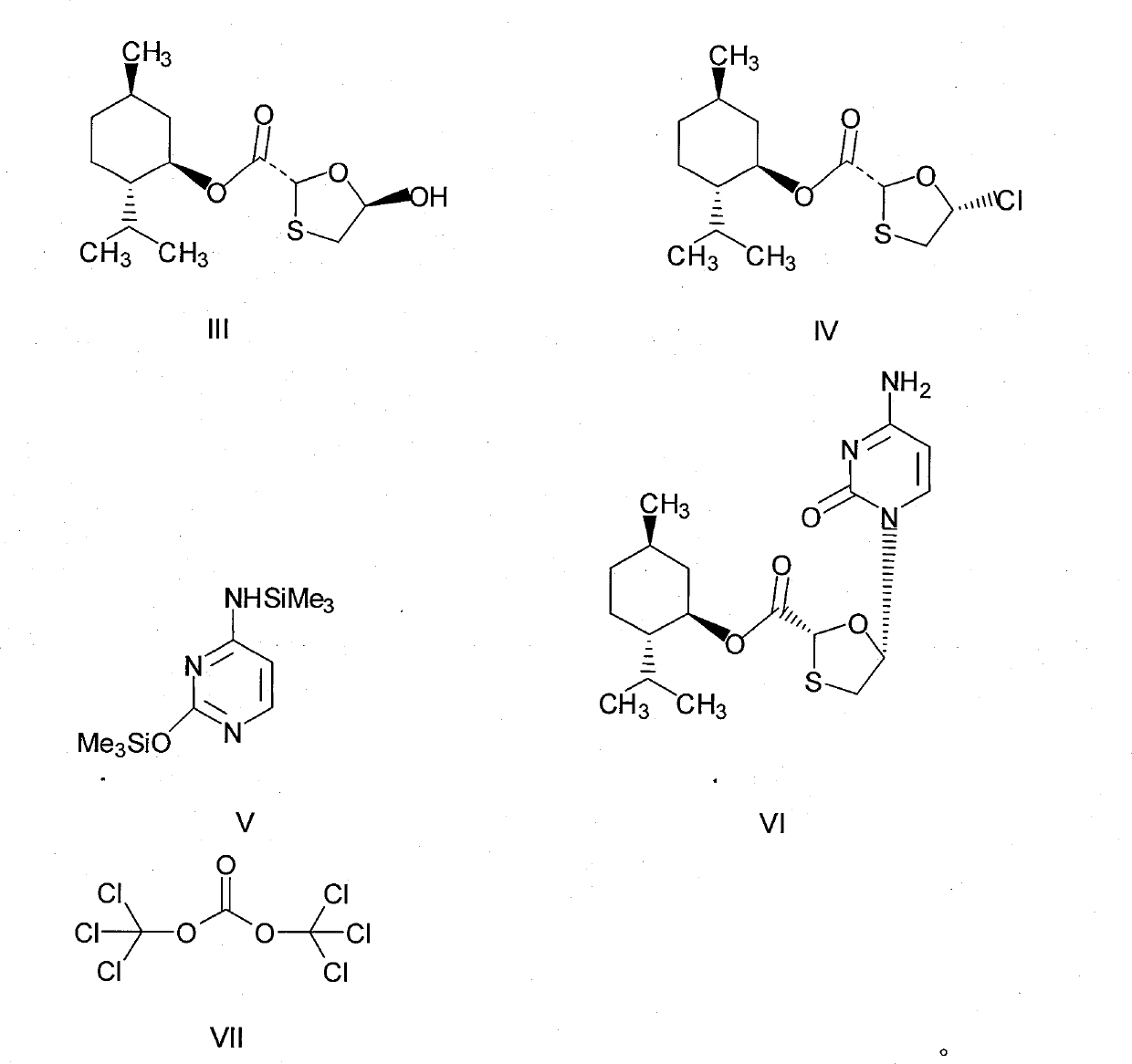
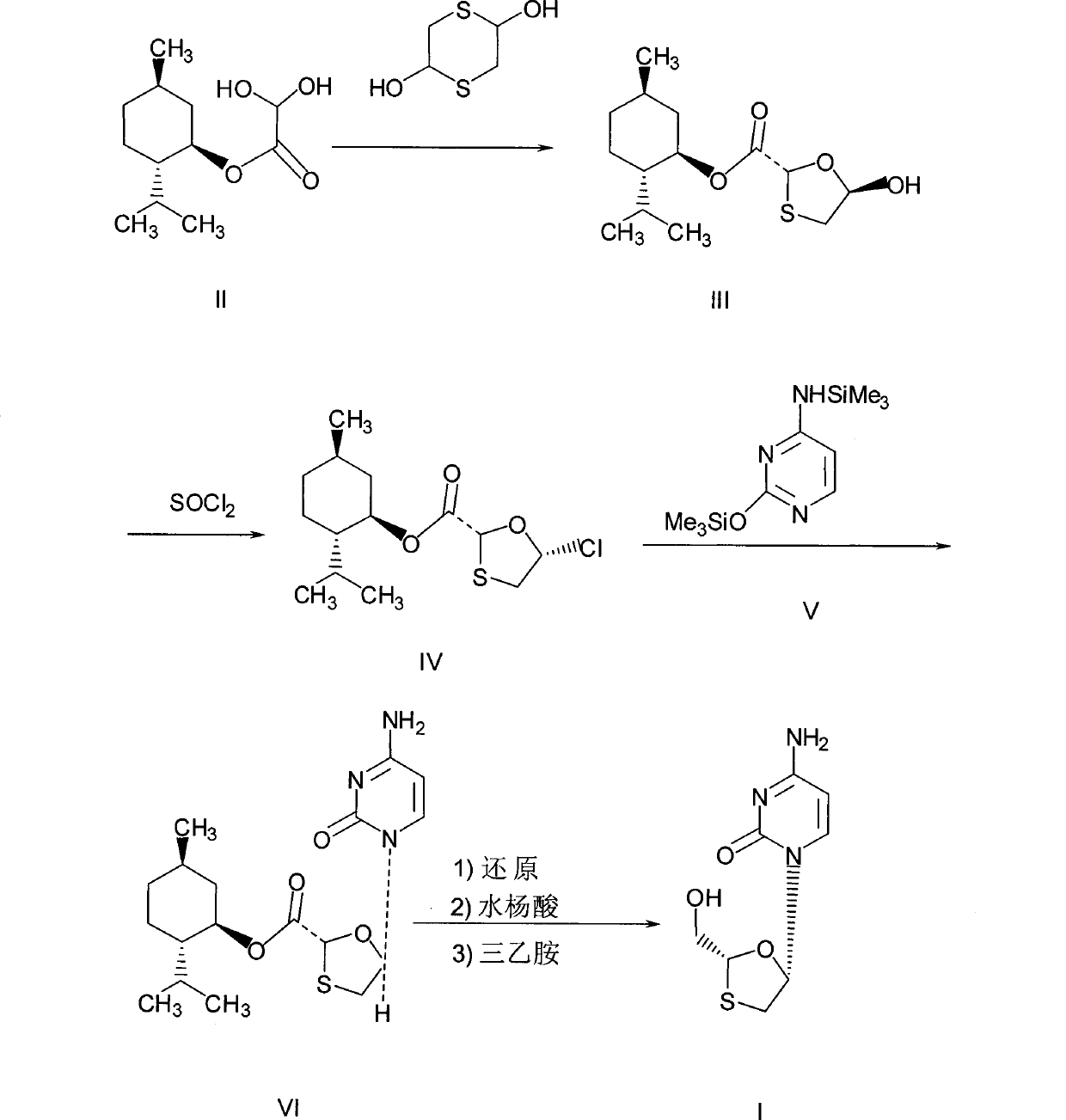
[0027]Preparation of chlorinated compounds: in a 250mL three-necked flask, add (2R, 5R)-5-hydroxyl-1,3-oxathiolane-2-carboxylic acid-L-menthyl ester (compound III) 28.8 grams (0.1mol), N,N-dimethylformamide 7.3g, 100mL dichloromethane, and keep stirring until compound III dissolves. Cool to about 0°C, control the temperature at 5-10°C, add dropwise a mixed solution of 11.9 grams (0.04mol) of bis(trichloromethyl)carbonate in 50mL of dichloromethane, and slowly heat up to 30-35°C, keep warm for 2 hours. The resulting reaction solution is a chloride (compound IV) solution, ready for use.
[0028] Preparation of N,O-bis(trimethylsilyl)cytosine: In a 500mL three-necked flask, add 11.1 grams (0.1mol) of cytosine, 24.5 grams of hexamethyldisilazane, 100mL of toluene, 3 drops of methanesulfonic acid, Heated to reflux for 3 hours until the solution was clear and cooled slightly. This was a toluene solution of N,O-bis(trimethylsilyl)cytosine.
[0029] Preparation of (2R, 5S)-5-(cytos...
Embodiment 2
[0032] Preparation of chlorinated compounds: in a 250mL three-necked flask, add (2R, 5R)-5-hydroxyl-1,3-oxathiolane-2-carboxylic acid-L-menthyl ester (compound III) 28.8 grams (0.1mol), N,N-dimethylformamide 7.3g, 100mL dichloromethane, and keep stirring until compound III dissolves. Cool to about 0°C, control the temperature at 5-10°C, add dropwise a mixed solution of 14.9 grams (0.05mol) of bis(trichloromethyl)carbonate in 50mL of dichloromethane, and slowly heat up to 30-35°C, keep warm for 2 hours. The resulting reaction solution is a chloride (compound IV) solution, ready for use.
[0033] Preparation of N, O-bis(trimethylsilyl)cytosine: Same as Example 1.
[0034] Preparation of (2R, 5S)-5-(cytosine-1-yl)-1,3-oxathiolane-2-carboxylic acid-L-menthyl ester: same as Example 1
[0035] Post-treatment: Same as Example 1, to obtain 26.8 g of white solid product, HPLC purity: 99.02%, chiral purity: 99.15%, melting point: 218.0-218.9°C, yield: 70.34%.
Embodiment 3 1
[0036] Embodiment 3 one pot method
[0037] In a 500mL three-neck flask, add 11.1g (0.1mol) of cytosine, 24.5g of hexamethyldisilazane, 100mL of toluene, and 3 drops of methanesulfonic acid, and heat to reflux for 3 hours until the solution is clear. This is N,O- Bis(trimethylsilyl)cytosine in toluene. Cool slightly, add 7.3g (0.1mol) of N,N-dimethylformamide, 12g of triethylamine, at a temperature of about 15-20°C, slowly add the (2R,5R)-5-hydroxy -1,3-oxathiolane-2-carboxylic acid-L-menthyl ester (compound III) 28.8 grams (0.1mol) and bis(trichloromethyl)carbonate 11.9 grams (0.04mol) were dissolved in 150mL of dichloromethane mixed solution, after dripping, keep warm for 3 hours, then slowly raise the temperature to 40-45°C, and react for 8 hours. Stop responding.
[0038] Post-processing: Pour the reaction solution into a mixed solution containing 100 g of water, 12 g of triethylamine, and 60 g of n-hexane, stir for 8 hours, filter, and rinse the resulting solid with 10...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com