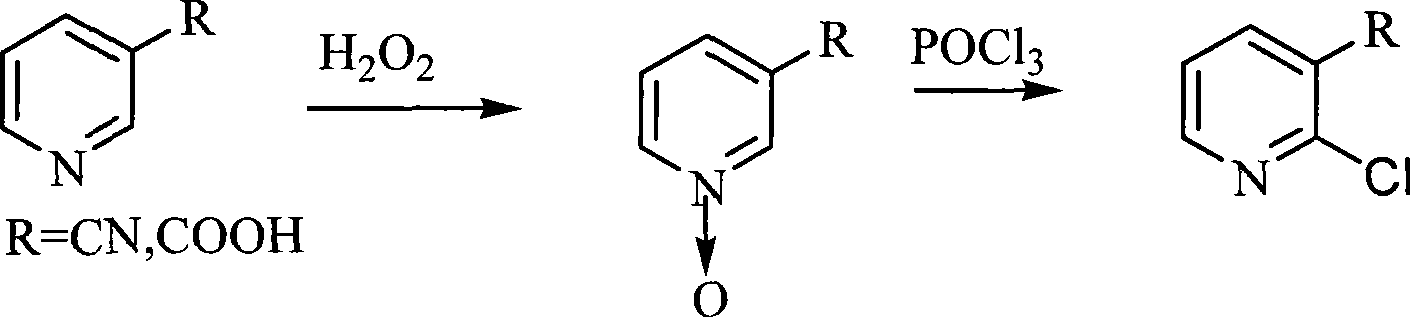
Synthesis of 2-chlorine apellagrin
A synthetic method, the technology of clonicotinic acid, which is applied in the field of synthesis of pharmaceutical and pesticide intermediates, can solve the problems of unavailable and high prices, and achieve the effects of high synthesis yield, reduced reaction time, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
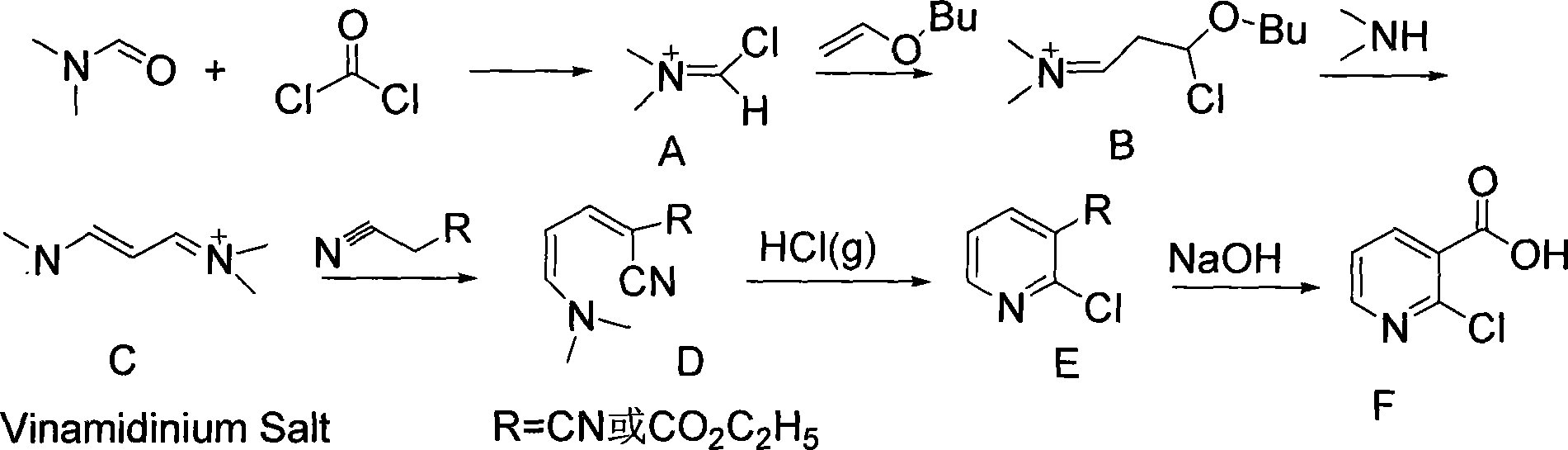
[0039] Synthesis of Compound A (Vilsmerier Reaction)
[0040] 36.5g of N,N-dimethylformamide and 50ml of 1,2-dichloroethane were cooled in an ice bath to 0°C, and 300ml of dichloroethane containing 50g of solid phosgene was added dropwise with stirring (about 90min). Then it was stirred at room temperature for 1 hour to obtain solid compound A (Vilsmerier reagent).
[0041] Synthesis of Compound B
[0042] Under cooling in a water bath, 50.0 g of vinyl n-butyl ether and 50 ml of 1,2 dichloroethane mixed solution was added dropwise to compound A above, then stirred at room temperature for 1 hour, and reacted at 65° C. for 15 minutes. The 1,2-dichloroethane was removed under reduced pressure to obtain compound B.
[0043] Preparation of Compound C (Vinamidinium Salts)
[0044] Aqueous dimethylamine solution was added dropwise to Compound B, reacted at 70°C for 1 hour, added toluene and refluxed to dehydrate until anhydrous, and cooled to obtain solid Compound C, about 126g. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com