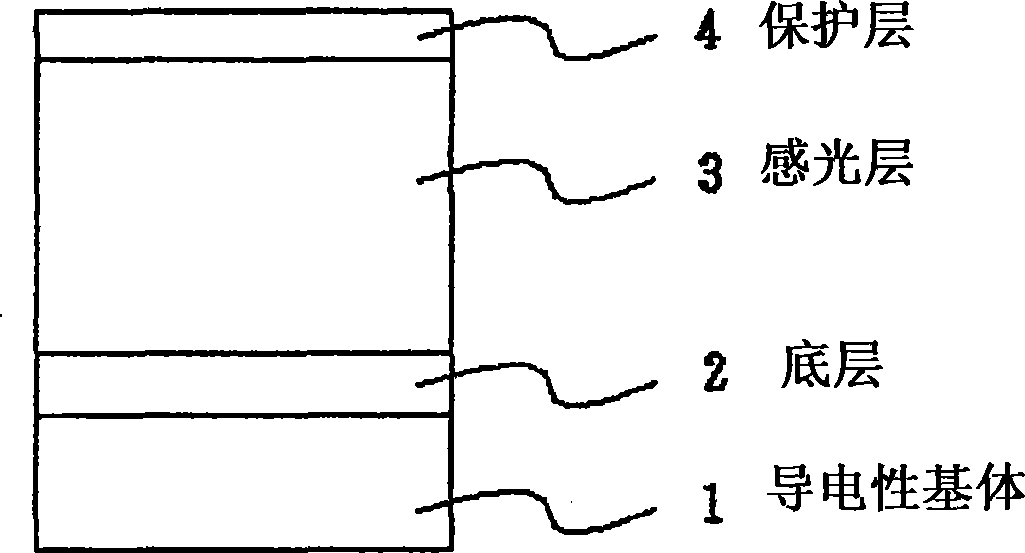
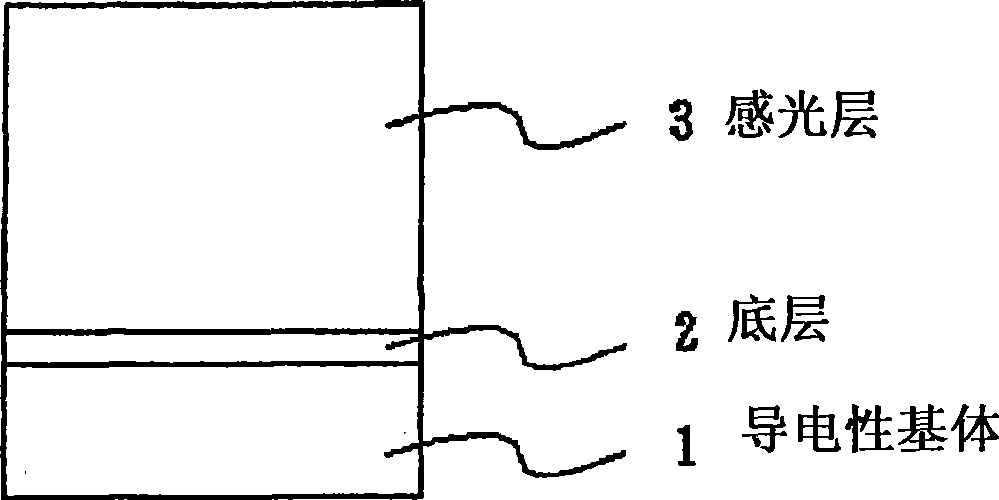
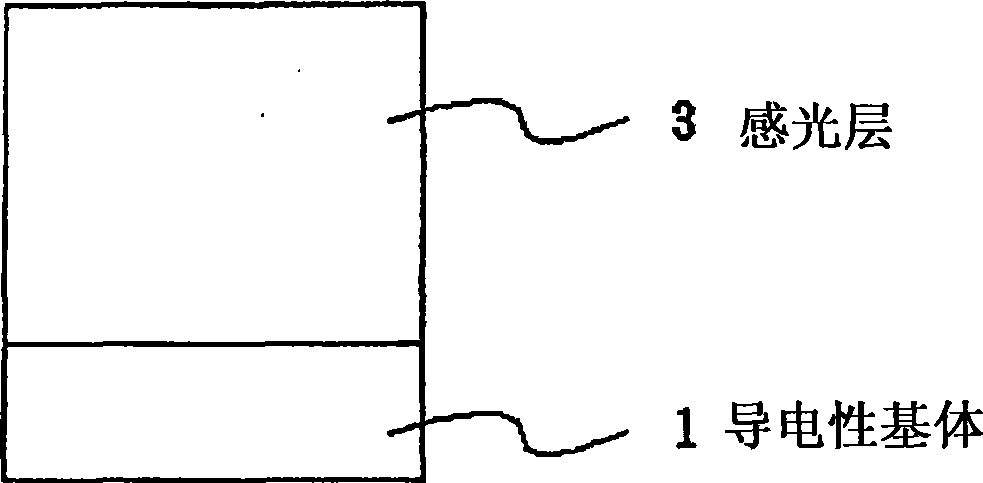
Quinone compound, electrophotographic photosensitive body and electrophotographic apparatus
A quinone compound, electrophotographic technology, applied in electrical recording process applying charge pattern, equipment, optics, etc., to achieve the effects of improving electrical properties, reducing notch wave, and excellent repeat stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131] Synthesis Example 1: Synthesis of the compound of the above-mentioned specific example (I-8)
[0132] (1) Synthesis of dihydrazone
[0133] 100.0 mL of concentrated hydrochloric acid was added to 28.0 g (124.2 mmol) of tin(II) chloride dihydrate and 737.3 mg (6.2 mmol) of tin, and heated and stirred until the tin was dissolved. 100.0 mL of concentrated hydrochloric acid was added to 10.0 g (31.1 mmol) of 2,2',5,5'-tetrachlorobenzidine, and stirred at room temperature for 1 hour until the amine crystals became a slurry. After cooling to -20°C, 18 ml of an aqueous solution of 4.5 g (65.2 mmol) of sodium nitrite was added dropwise over 30 minutes, followed by stirring for 1 hour. The above-mentioned tin chloride solution was cooled to 5° C., and added dropwise to the diazotization solution over 30 minutes. After the completion of the addition, the mixture was stirred for 1 hour, and the generated solid was separated by filtration with a glass filter, and washed with 10...
Embodiment 2
[0143] Synthesis Example 2: Synthesis of the compound of the above-mentioned specific example (I-21)
[0144] (1) Synthesis of dihydrazone
[0145] Using 10.0 g (31.2 mmol) of 2,2'-bistrifluoromethylbenzidine, it was synthesized in the same manner as in Synthesis Example 1-(1) to obtain 24.0 g (30.7 mmol) of the title compound.
[0146] Yield 98.2%, mp223℃~226℃
[0147] 1 H-NMR (500MHz, CDCl 3 )
[0148] δ1.48(s, 36H), δ5.38(s, 2H), δ7.18(d, J=8.5Hz, 2H), δ7.23(dd, J=8.5Hz, 2.4Hz, 2H), δ7 .42(d, J=2.4Hz, 2H), δ7.51(s, 4H), δ7.61(brs, 2H), δ7.72(s, 2H)
[0149] MS (m / z): 783, 551, 319, 190
[0150] (2) Synthesis of the compound of specific example (I-21)
[0151] Using 5.00 g (6.39 mmol) of the hydrazone compound obtained in Synthesis Example 2-(1), it was synthesized in the same manner as in Synthesis Example 1-(2) to obtain 3.18 g (4.08 mmol) of the title compound.
[0152] Yield 63.9%, mp149℃~154℃
[0153] 1 H-NMR (500MHz, CDCl 3 )
[0154] δ1.37(s, 18H), δ1.39...
Embodiment 3
[0156] Synthesis Example 3: Synthesis of the compound of the above specific example (I-28)
[0157] (1) Synthesis of 2,2'-bistrifluoromethyl-5,5'-dibromobenzidine
[0158] Under a nitrogen atmosphere, 20.0 g (62.5 mmol) of 2,2'-bistrifluoromethylbenzidine was dissolved in 100 mL of ethanol, and 21.0 g (131.2 mmol) of bromine was added dropwise over 1 hour under ice-cooling. After stirring at room temperature for 1 hour, toluene was added, and the organic phase was washed three times with water, and then washed twice each with saturated sodium bicarbonate water and water. After concentration, it was recrystallized from hexane / toluene to obtain 11.5 g (24.1 mmol) of the title compound.
[0159] Yield 38.5%, mp154℃~157℃
[0160] 1 H-NMR (500MHz, CDCl 3 )
[0161] δ4.33(s, 4H), δ7.05(s, 2H), δ7.32(s, 2H)
[0162] MS (m / z): 478, 298
[0163] (2) Synthesis of dihydrazone
[0164] Using 5.0g (10.5mmol) of 2,2'-bistrifluoromethyl-5,5'-dibromobenzidine, synthesized in the sam...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com