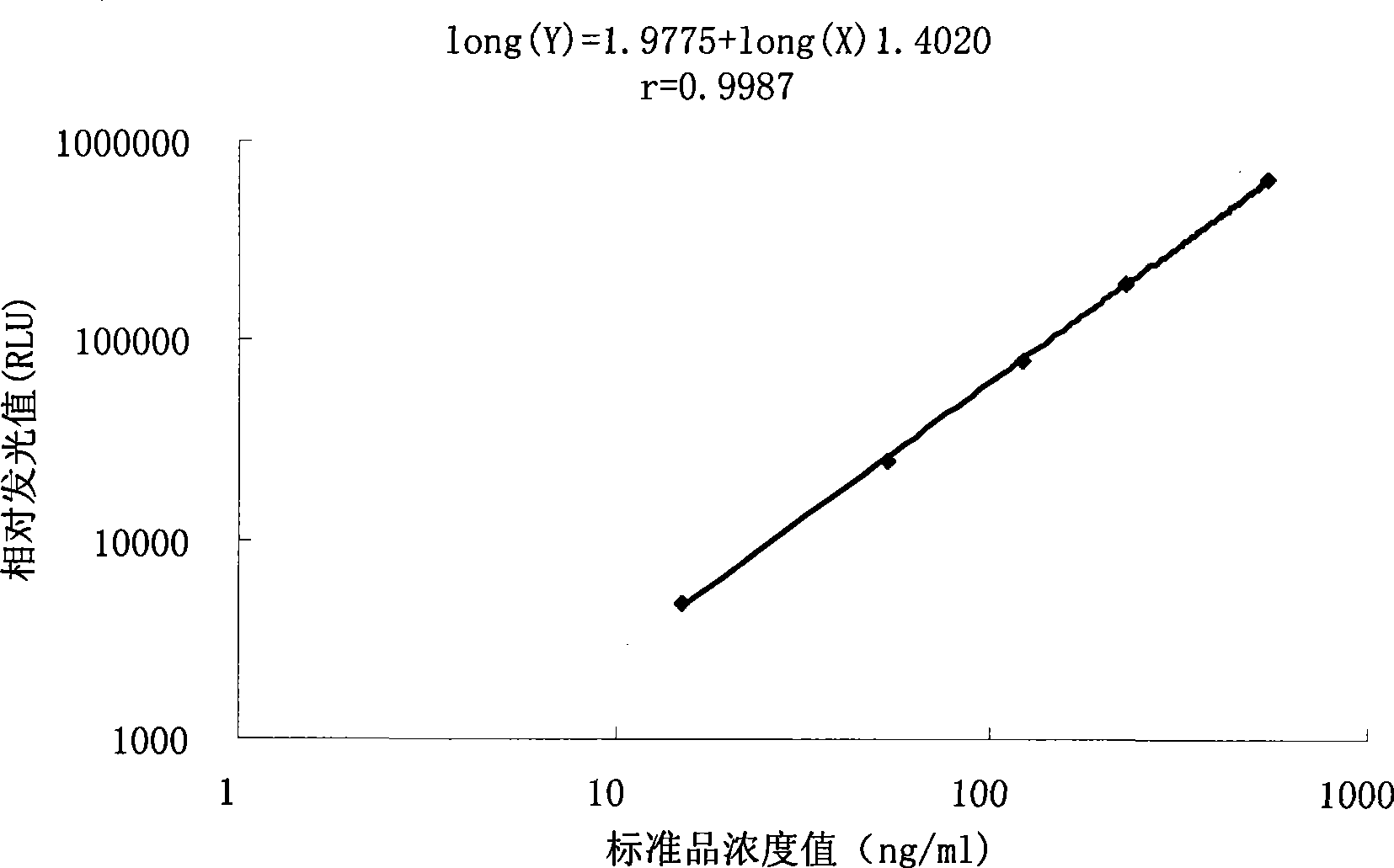
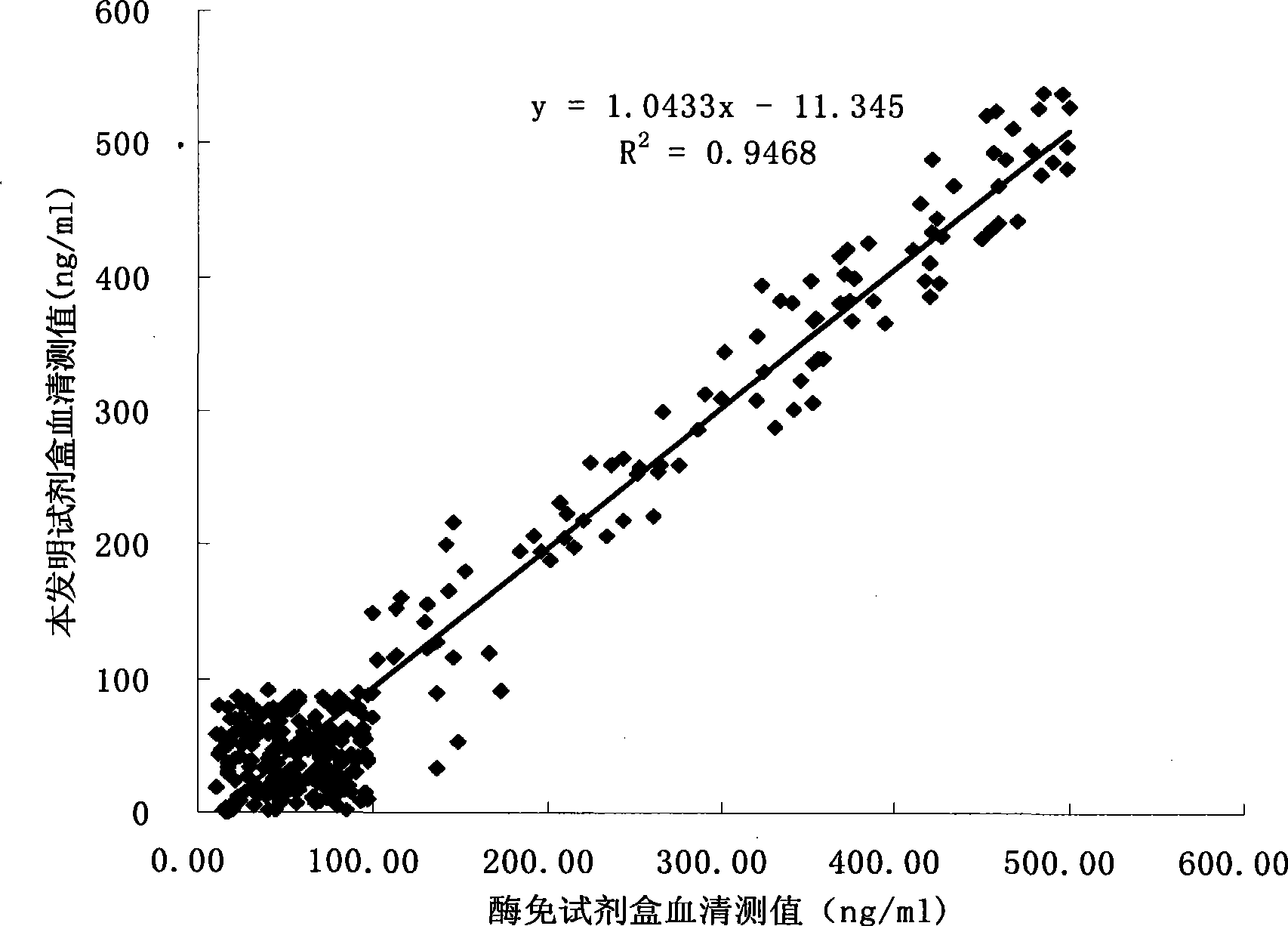
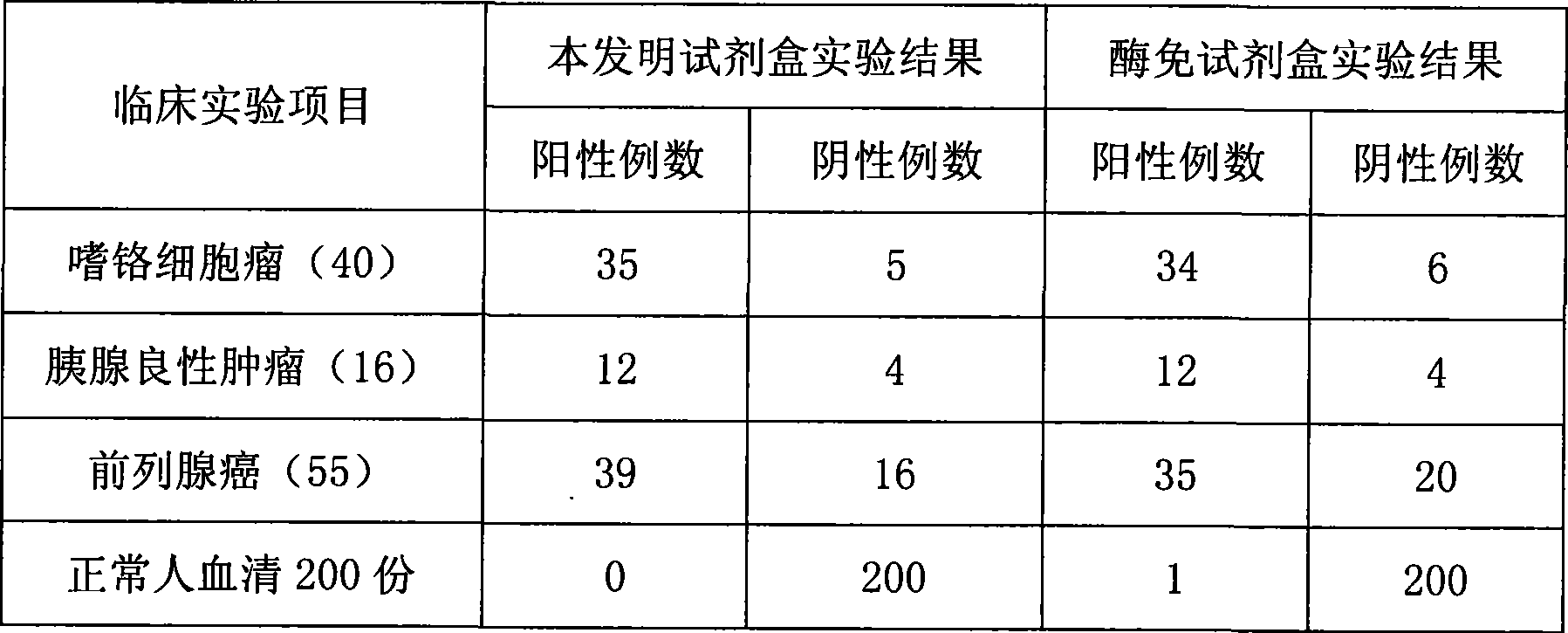
Chromogranin A chemiluminescence immune analysis quantitative determination reagent kit and preparing method thereof
A chemiluminescence immunity and chemiluminescence technology, which is applied in the field of immunoanalysis medicine, can solve problems such as narrow detection range, false positives, and failure to meet clinical requirements, and achieve high sensitivity and stability, good specificity and accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Preparation of Chromogranin A Chemiluminescent Immunoassay Quantitative Assay Kit of the Present Invention Using Alkaline Phosphatase System
[0028] 1. Preparation of alkaline phosphatase-labeled antibody
[0029] The CgA-specific antibody was conjugated with alkaline phosphatase by classical glutaraldehyde cross-linking method, fully dialyzed with PBS buffer, and an equal volume of glycerol was added to the conjugate solution and stored in a low-temperature refrigerator (-20°C). Dilute with 20% bovine serum enzyme diluent when used again. The optimal working concentration range of the enzyme-labeled antibody is 1:5000-10000 through optimization experiments.
[0030] The formula of the 20% bovine serum enzyme dilution used is:
[0031] NaH2PO4·2H2O 1.28mM 0.2g
[0032] Na2HPO4·12H2O 8.10mM 2.9g
[0033] NaCl 150mM 8.8g
[0034] Newborn Calf Serum 20% 200ml
[0035] Proclin300 0.1% 1.0ml
[0036] Food Red 0.05‰ 1.0ml
[0037] Dilute to 1000mL with deio...
Embodiment 2
[0074] Example 2 Application of horseradish peroxidase system to prepare chromogranin A chemiluminescent immunoassay quantitative assay kit of the present invention
[0075] 1. Preparation of horseradish peroxidase-labeled antibody
[0076] Dissolve 4.4mg HRP in 1mL distilled water, add 0.4mL sodium periodate (50mmol / L) and stir at room temperature for 20min, dialyze through 1mmol / L sodium acetate buffer solution, pH value is 4.4, add 8mg chromogranin A specific antibody, Stir for 2h, and finally use 200mmol / L NaBH 4 For reduction, after dialysis with 0.02M PBS buffer, add an equal volume of glycerol, and store below -20°C.
[0077] 2. Preparation of chemiluminescence substrate
[0078] 1. Chemiluminescent Substrate A Solution
[0079] Luminol 10mM 1.7716g
[0080] 4-Hydroxybiphenyl 0.3mM 0.051g
[0081] 4-iodophenylboronic acid 0.05mM 0.012g
[0082] Boric acid 11.4g
[0083] Borax 4.9g
[0084] Double distilled water fixed solution 1000mL
[0085] Adjust pH to 8.0~1...
Embodiment 3~4
[0103] Examples 3-4 Preparation of Chromogranin A Chemiluminescence Immunoassay Quantitative Assay Kit of the present invention
[0104] Except that plastic beads were used as the carrier, the rest were the same as those in Examples 1 and 2 for preparing the quantitative assay kit of the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com