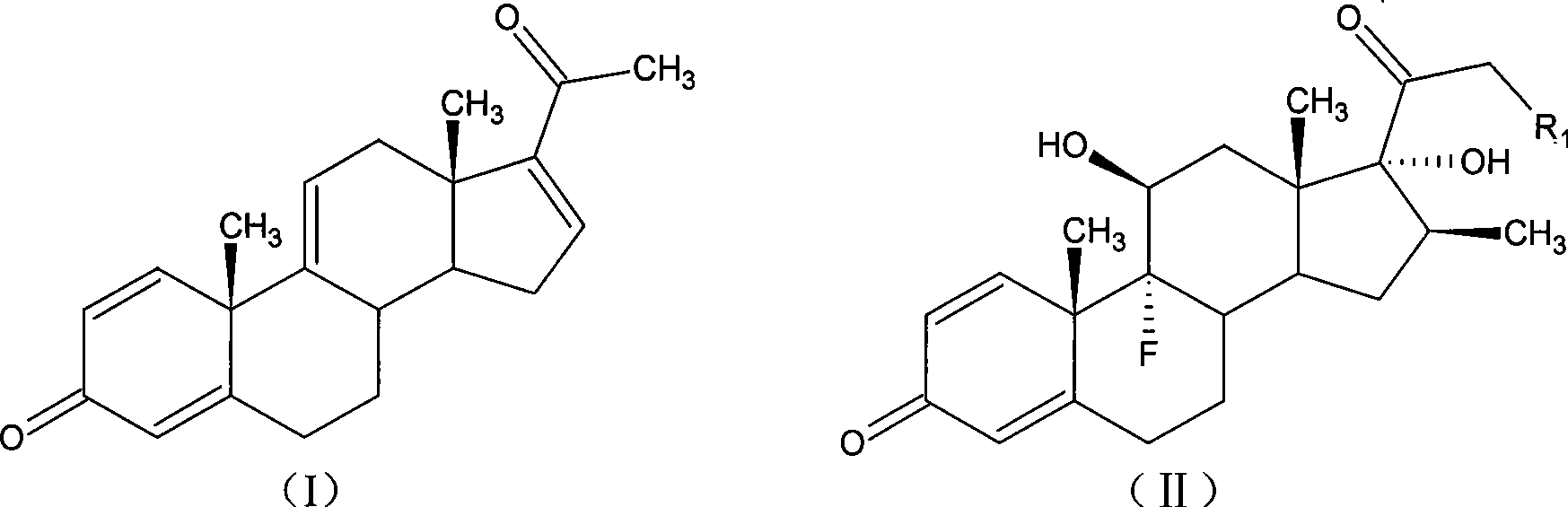
Method for preparing betamethasone and series products thereof
A technology for betamethasone and a compound is applied in the field of preparation of steroid compounds, which can solve problems such as long process routes, and achieve the effects of easy availability of raw materials, concise routes, and reduced production costs and industrialization conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] The preparation of embodiment one betamethasone acetate
[0062] Grignard reaction: 17α-hydroxy-16β-methyl-1,4,9-triene-pregna-3,20-dione;
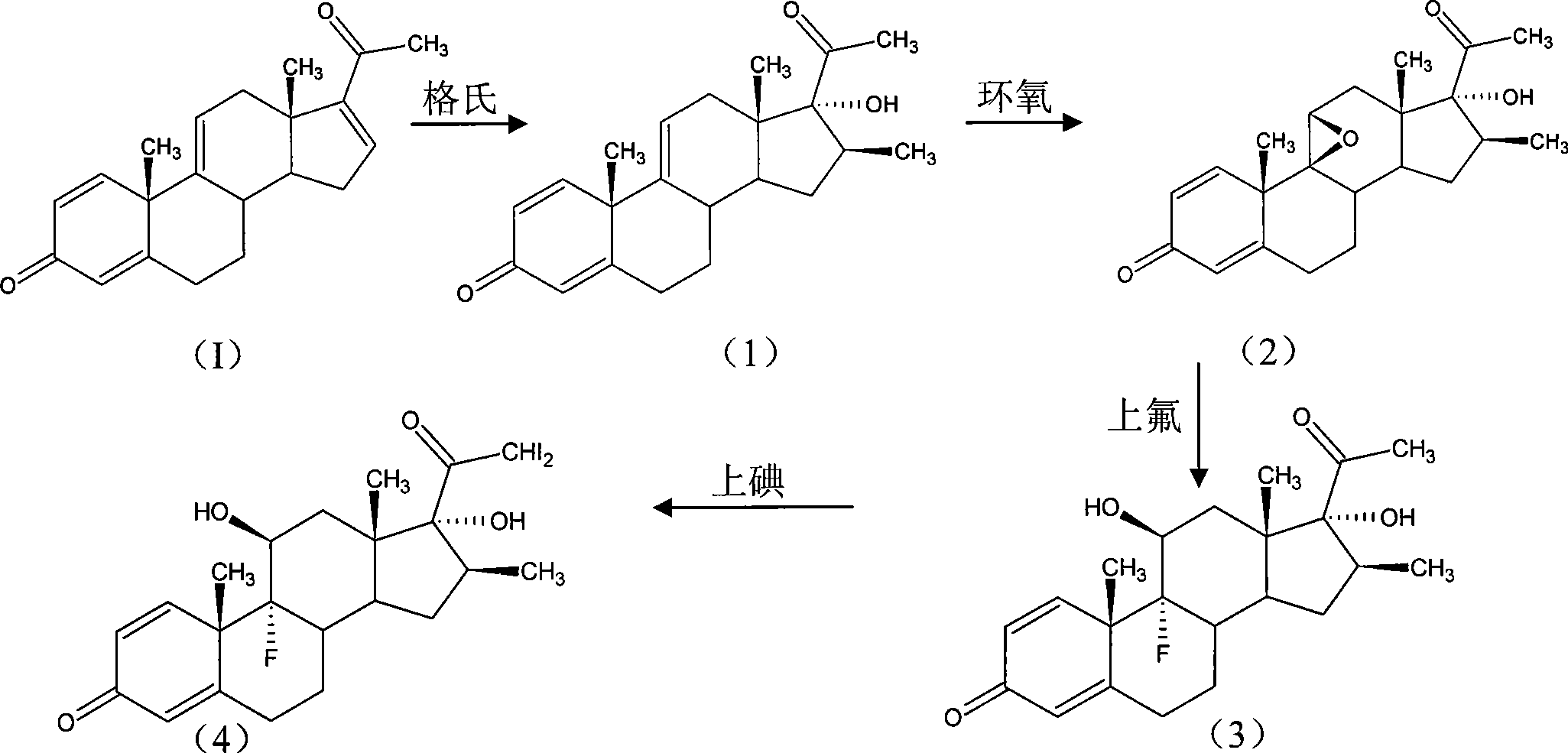
[0063] Preparation of Grignard reagent: add 60ml tetrahydrofuran to the reaction bottle, add 6g magnesium flakes and 8g iodine in turn, adjust the temperature to 50±5°C, add 20g methyl bromide and 80ml tetrahydrofuran solution dropwise, control the temperature at 40±5°C, add After incubation for 1 hour, the Grignard reagent was obtained.
[0064] 12 g of 1,4,9,16-tetraene-pregna-3,20-dione and 50 ml of tetrahydrofuran were added to the reaction flask. Raise the temperature to 50±5°C, add the Grignard reagent dropwise until the sampling HPLC test is qualified. Add 20ml of ammonium chloride, add 30ml of hydrogen peroxide and react at 20±5°C for 2 hours until the sampling HPLC test is qualified. Cool down to 0±5°C, filter, and dry to obtain 10 g of Grignard (1).
[0065] Epoxy reaction: 9β, 11β-epoxy-17α-hydroxy-16β-methyl-1,4-die...
Embodiment 2
[0073] The preparation of embodiment two betamethasone acetate
[0074] Grignard reaction: 17α-hydroxy-16β-methyl-1,4,9-triene-pregna-3,20-dione;
[0075] Preparation of Grignard reagent: add 60ml tetrahydrofuran to the reaction bottle, add 6g magnesium flakes and 8g iodine in turn, adjust the temperature to 50±5°C, add 20g methyl bromide and 80ml tetrahydrofuran solution dropwise, control the temperature at 40±5°C, add After incubation for 1 hour, the Grignard reagent was obtained.
[0076] 12 g of 1,4,9,16-tetraene-pregna-3,20-dione and 50 ml of tetrahydrofuran were added to the reaction flask. Raise the temperature to 50±5°C, add the Grignard reagent dropwise until the sampling HPLC test is qualified. Add 20ml of ammonium chloride, add 30ml of sodium peroxide solution and react at 30±5°C for 2 hours until the sampling HPLC test is qualified. Cool down to 0±5°C, filter, and dry to obtain 10 g of Grignard (1).
[0077] Iodine reaction: 21-diiodo-17α-hydroxy-16β-methyl-1,4...
Embodiment 3
[0085] The preparation of embodiment three betamethasone
[0086] Grignard reaction: 17α-hydroxy-16β-methyl-1,4,9-triene-pregna-3,20-dione;
[0087] The method is the same as the Grignard reaction method in Example 1 to obtain the Grignard compound (1).
[0088] Epoxy reaction: 9β, 11β-epoxy-17α-hydroxy-16β-methyl-1,4-diene-pregna-3,20-dione;
[0089] The method is the same as the epoxy reaction method in Example 1 to obtain 10.5g epoxy (2).
[0090] Fluorine reaction: 9α-fluoro-11β, 17α-dihydroxy-16β-methyl-1,4-diene-pregna-3,20-dione;
[0091] The method is the same as the fluorine-on reaction method in Example 1 to obtain 10.8 g of fluorine-on compound (3).
[0092] Iodine reaction: 9α-fluoro-21-diiodo-11β, 17α-dihydroxy-16β-methyl-1,4-diene-pregna-3,20-dione;
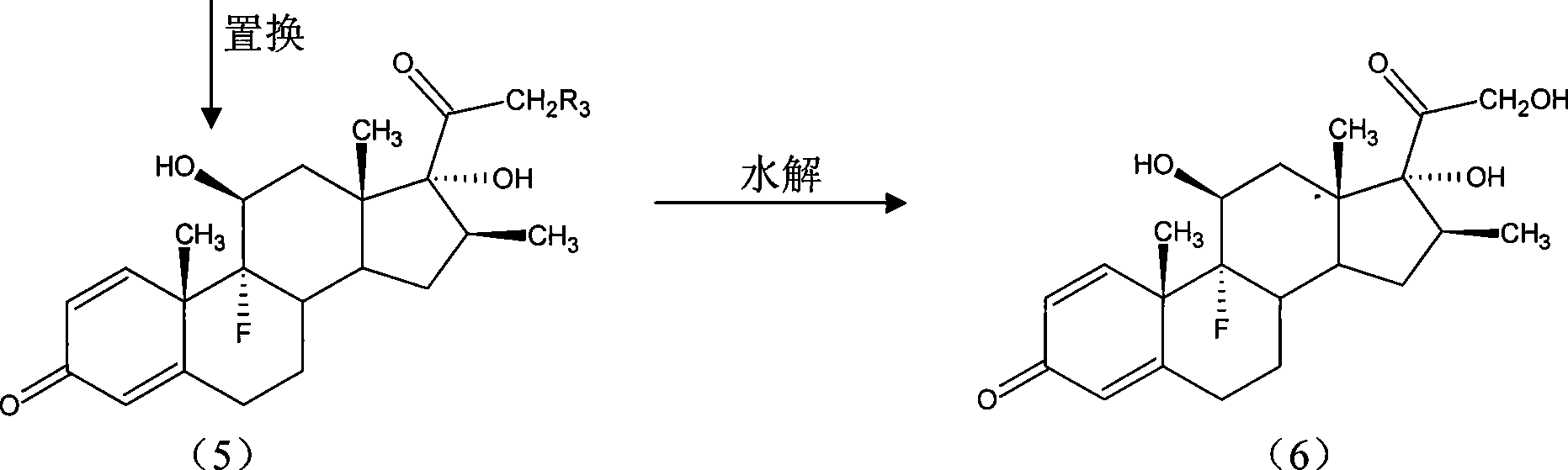
[0093] The method is the same as the iodine reaction method in Example 1 to obtain the wet product iodide (4). This product is unstable and does not need to be dried. The storage time should not be too long, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com