Process and special equipment for producing trifluoro ethylene with catalytic hydrogenolysis of chlorotrifluoroethylene
A technology of chlorotrifluoroethylene and trifluoroethylene, which is applied in the field of catalytic hydrogenolysis of chlorotrifluoroethylene to prepare trifluoroethylene, can solve the problems of low output per unit reaction volume, low conversion rate and selectivity, and large equipment investment, and achieves Process method and equipment optimization, high conversion rate and selectivity, and obvious economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 The temperature is 110°C, and the pulse frequency is 1 / 30min -1
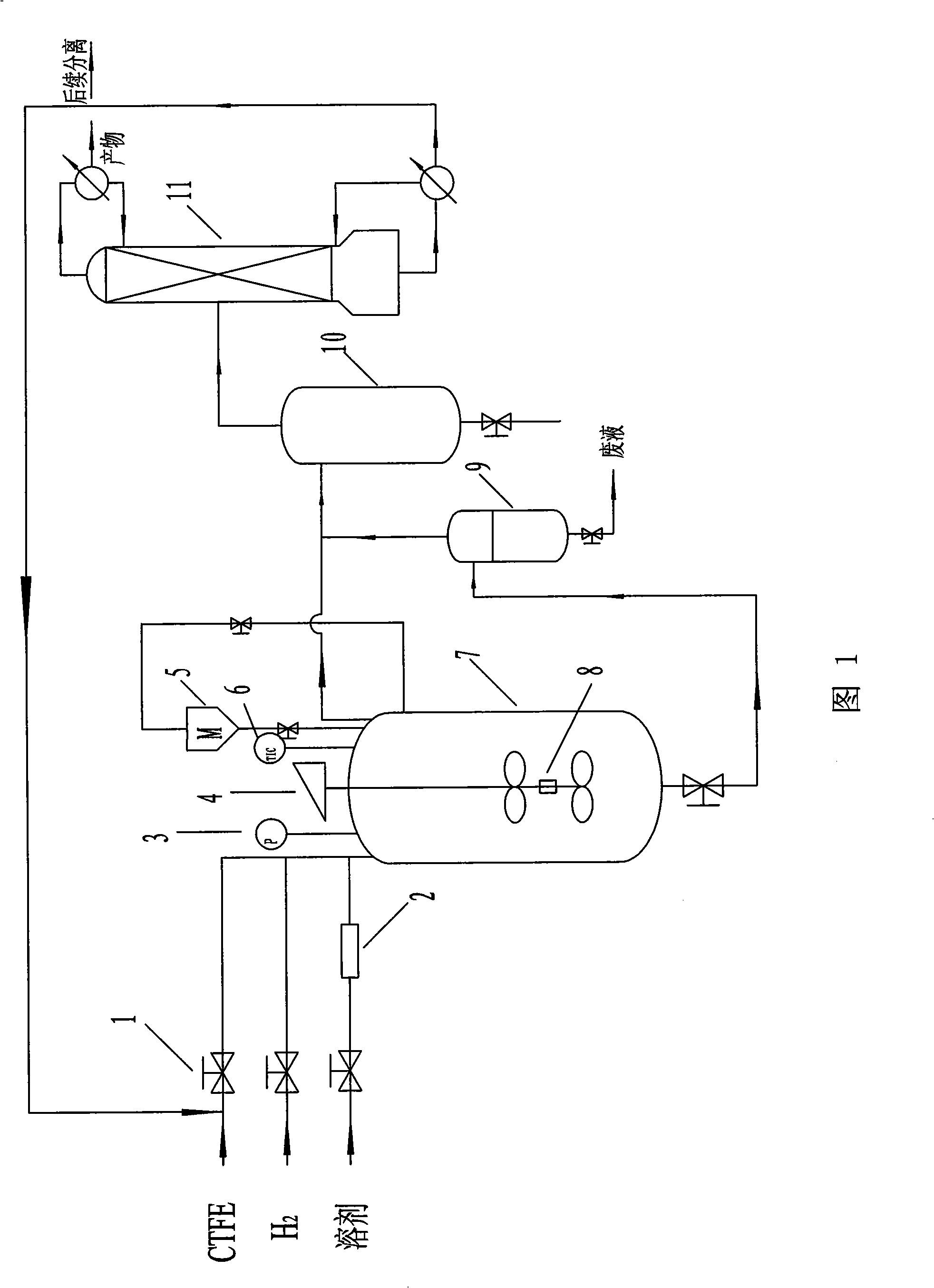
[0040] Through the automatic regulating valve 1, through the liquid flow meter 2, into the three stainless steel autoclaves 7 with a volume of 1 L in series, 1.25 mol of raw material chlorotrifluoroethylene, 0.625 mol of hydrogen, 25 g of HCl acceptor zinc powder and The aqueous solution 350ml of reaction solvent 25% ethanol, raw material is fully stirred and mixed by motor agitator 4 in reactor 7, is stirred and reacted at 110 ℃ by temperature controller 6 control temperature, the 5%Pd in the shaft plate frame 8 of stirring paddle Hydrogenolysis reaction occurs under the action of a catalyst of 0.7g / C; observe the pressure gauge 3, according to the pulse frequency, pulse feeding and discharging once every 30min, the feeding amount of hydrogen and chlorotrifluoroethylene is 1 / 10 of the gas pressure in the kettle , the molar ratio of hydrogen to chlorotrifluoroethylene is 0.5; with the feedin...
Embodiment 2
[0041] Example 2 The temperature is 100°C, and the pulse frequency is 1 / 60min -1
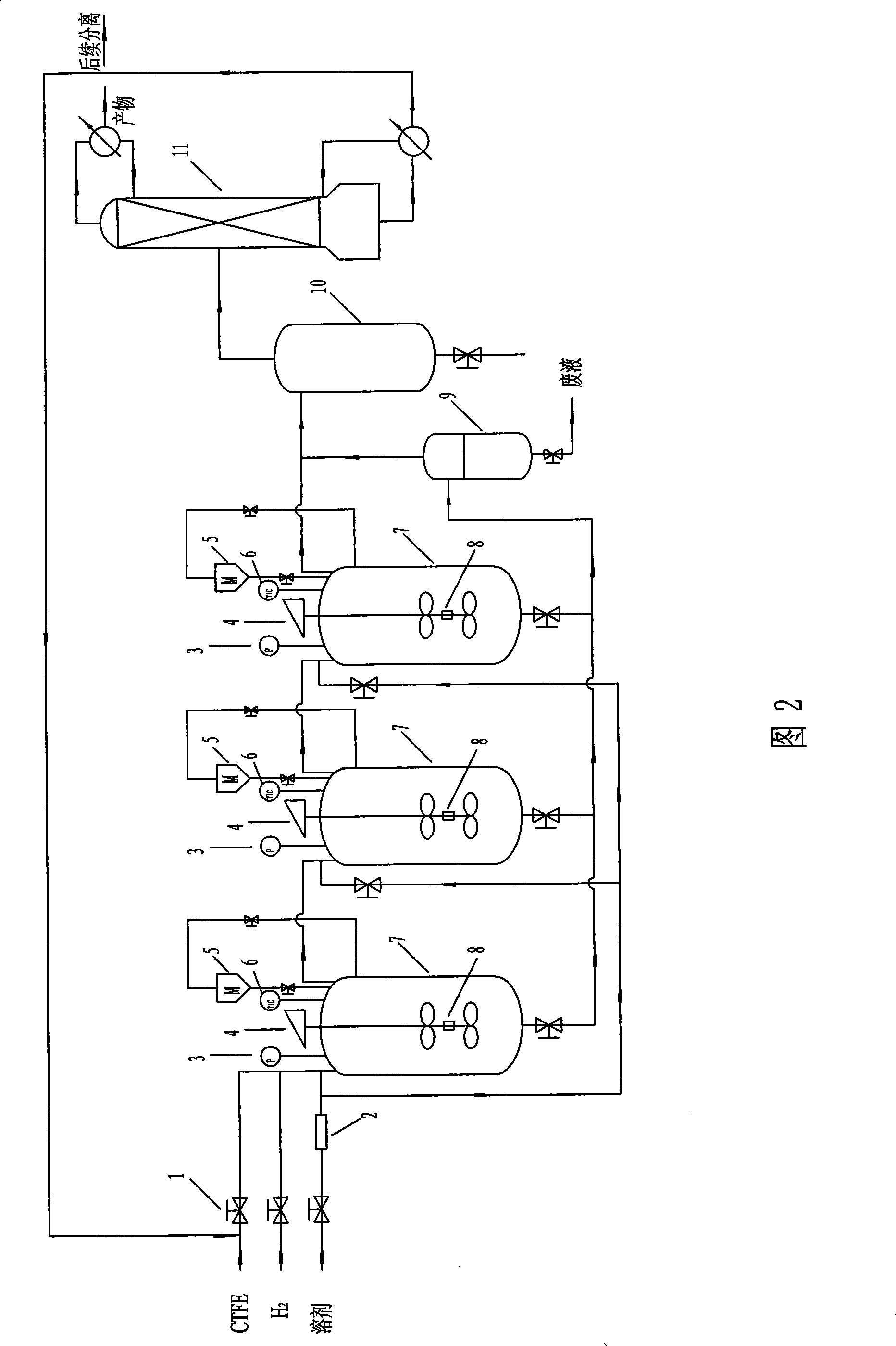
[0042] The process flow is the same as that in Example 1, and sequentially add 0.6 g of 5% Pd / C catalyst, 0.09375 mol of ammonia, 200 ml of solvent DMF, 0.75 mol of chlorotrifluoroethylene raw material, and 0.375 mol of hydrogen into two stainless steel autoclaves connected in series with a volume of 1 L. , stirring and reacting at 100°C, pulse feeding and discharging once every 60min, the feeding amount of hydrogen and chlorotrifluoroethylene is 1 / 8 of the gas pressure in the kettle, and the molar ratio of hydrogen to chlorotrifluoroethylene is 0.6; Taking the feeding amount of chlorotrifluoroethylene as a reference, the feeding amount of the hydrogen chloride acceptor is 0.75 times of the molar weight of chlorotrifluoroethylene, and the feeding amount of the solvent is 200ml / 1mol chlorotrifluoroethylene. Continuous operation for 24h consumes The raw material is 13mol of chlorotrifluoroethylen...
Embodiment 3
[0043] Example 3 The temperature is 200°C, and the pulse frequency is 1 / 10min -1
[0044] The technological process is the same as in Example 1, and successively add 0.625 g of 5% Pt / C catalyst, 15 mol of pyridine, 500 ml of solvent cyclohexane, 1.5 mol of chlorotrifluoroethylene raw material, hydrogen 2.7mol, stirring reaction at 200°C, pulse feeding and discharging once every 10min, the feeding amount of hydrogen and chlorotrifluoroethylene is 1 / 7 of the gas pressure in the kettle, and the molar ratio of hydrogen to chlorotrifluoroethylene is 0.7; With reference to the feeding amount of chlorotrifluoroethylene, the feeding amount of hydrogen chloride acceptor is 0.8 times of the molar weight of chlorotrifluoroethylene, and the feeding amount of solvent is 300ml / 1mol chlorotrifluoroethylene, continuous operation 24h, A total of 13 mol of raw material chlorotrifluoroethylene was consumed. Gas chromatographic analysis shows that the selectivity of trifluoroethylene is 95%, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com