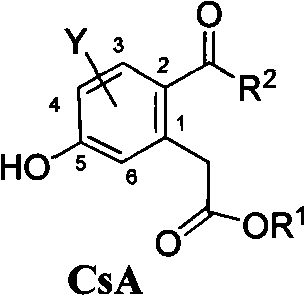
Bihydroxyl-2-acyl benzene acetic acid ester, producing method and uses thereof
A technology of acyl phenylacetate and methyl dihydroxybenzoate, which is applied in the field of bishydroxy-2-acyl phenylacetate and its preparation, and can solve the problems that have not yet been reported on the synthesis of CytosporoneB and its homologues, affecting research and application Progress, limited quantities, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
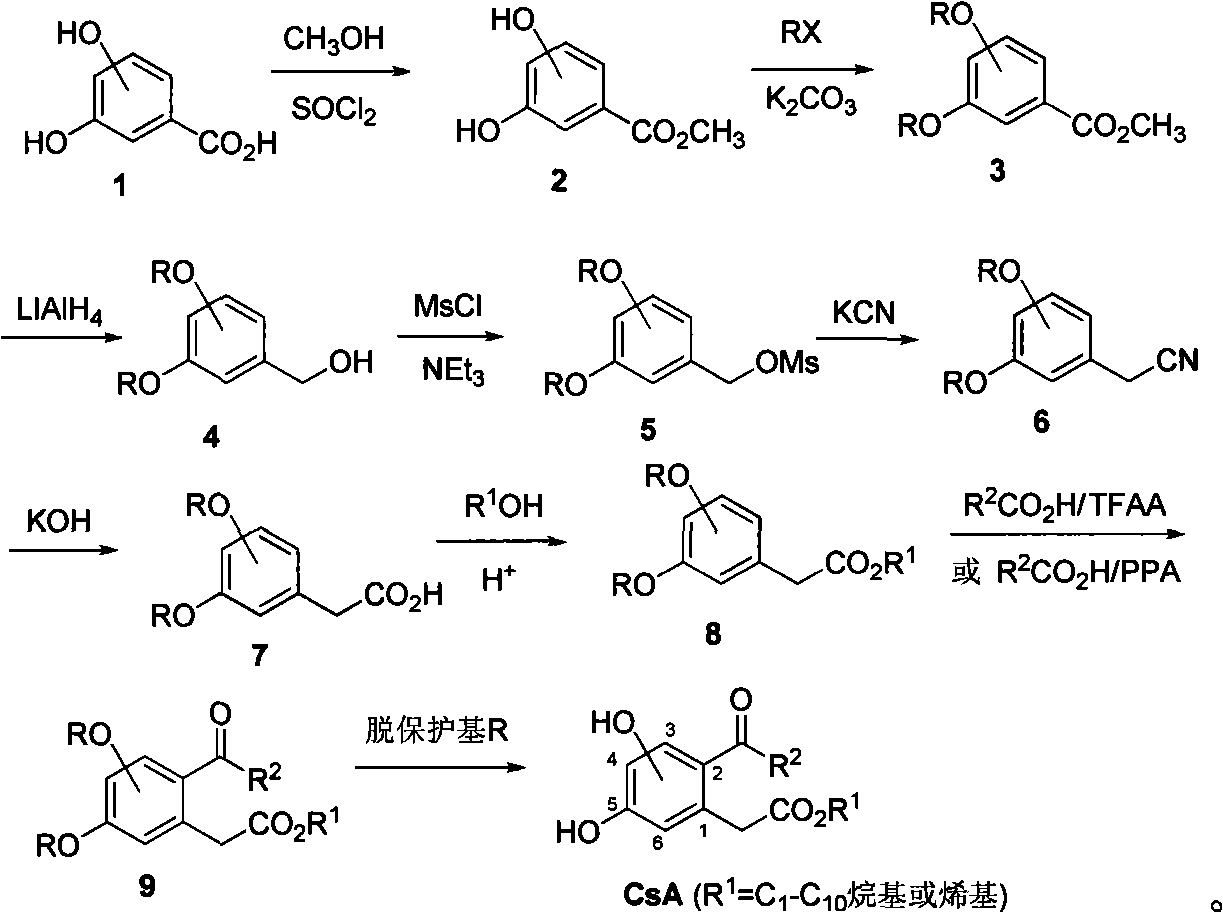
Examples
Embodiment 1
[0057] 3,5-dihydroxy-2-nonanoyl-phenylacetic acid ethyl ester (compound CsA2, R 1 =C 2 h 5 , R 2 =n-C 8 h 17 )
[0058] 1) Methyl 3,5-dihydroxybenzoate (compound 2)
[0059] 3,5-Dihydroxybenzoic acid (2.4mmol) was dissolved in 5mL of methanol, thionyl chloride (3.6mmol) was added, and the mixture was stirred at room temperature for 3h. Evaporate methanol and excess thionyl chloride, add water, extract with ethyl acetate, combine organic extracts, wash with sodium bicarbonate solution, anhydrous MgSO 4 After drying and concentration under reduced pressure, the residue was separated by column chromatography to obtain methyl 3,5-dihydroxybenzoate with a yield of 95%.
[0060] 2) Methyl 3,5-dibenzyloxybenzoate (compound 3, R=CH 2 C 6 h 5 )
[0061] Methyl 3,5-dihydroxybenzoate (20 mmol) was dissolved in 30 mL of acetone, benzyl bromide (43 mmol) and powdered anhydrous K 2 CO 3 (85 mmol), heated to reflux under stirring for 4.5 h, filtered, evaporated the solvent under...
Embodiment 2
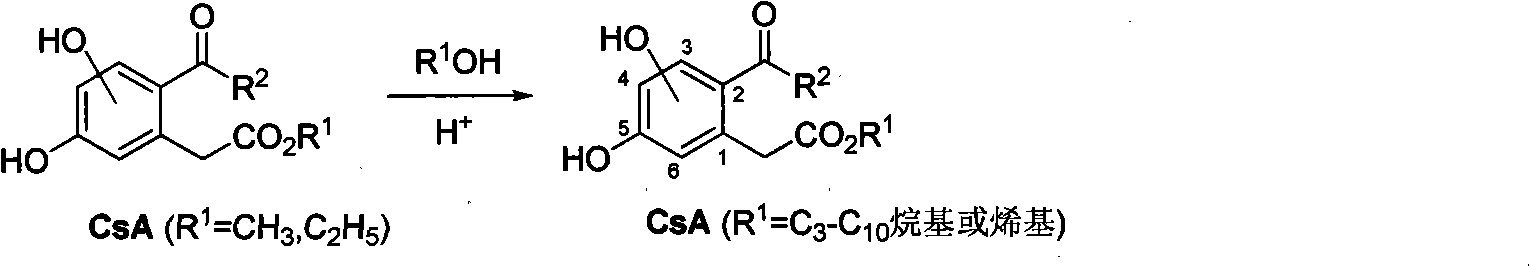
[0077] 3,5-dihydroxy-2-octanoyl-phenylacetic acid ethyl ester (compound CsA1 (cytosporone B), R 1 =C 2 h 5 , R 2 =n-C 7 h 15) Preparation of CsA1 from the intermediate product 3,5-dibenzyloxyethyl phenylacetate 8 (R=CH 2 C 6 h 5 , R 1 =C 2 h 5 )start.
[0078] 1) 3,5-dibenzyloxy-2-octanoyl-phenylacetic acid ethyl ester (compound 9, R=CH 2 C 6 h 5 , R 1 =C 2 h 5 , R 2 =n-C 7 h 15 )
[0079] Ethyl 3,5-dibenzyloxyphenylacetate (1 mmol) was dissolved in trifluoroacetic anhydride (3.8 mL), n-octanoic acid (1.05 mmol) was added, and the mixture was stirred at room temperature for 48 h. Add 2 mL of sodium bicarbonate solution, extract with ether, combine the organic extracts, wash with saturated sodium chloride solution, anhydrous MgSO 4 Dry, concentrate under reduced pressure, and purify by column chromatography to obtain ethyl 3,5-dibenzyloxy-2-octanoyl-phenylacetate with a yield of 74%.
[0080] 2) 3,5-dihydroxy-2-octanoyl-phenylacetic acid ethyl ester (compo...
Embodiment 3
[0083] 3,5-Dihydroxy-2-propionyl-phenylacetic acid ethyl ester (compound CsA5, R 1 =C 2 h 5 , R 2 =C 2 h 5 ) Preparation of CsA5 From the intermediate product 3,5-dibenzyloxyethyl phenylacetate 8 (R=CH 2 C 6 h 5 , R 1 =C 2 h 5 )start.
[0084] 1) 3,5-dibenzyloxy-2-propionyl-phenylacetic acid ethyl ester (compound 9, R=CH 2 C 6 h 5 , R 1 =C 2 h 5 , R 2 =C 2 h 5 )
[0085] Ethyl 3,5-dibenzyloxyphenylacetate (0.8 mmol) was dissolved in trifluoroacetic anhydride (2.5 mL), propionic acid (0.96 mmol) was added, and the mixture was stirred at room temperature for 48 h. Add 1mL sodium bicarbonate solution, extract with ether, combine the organic extracts, wash with saturated sodium chloride solution, anhydrous MgSO 4 Dry, concentrate under reduced pressure, and purify by column chromatography to obtain ethyl 3,5-dibenzyloxy-2-propionyl-phenylacetate with a yield of 76%.
[0086] 2) 3,5-dihydroxy-2-propionyl-phenylacetic acid ethyl ester (compound CsA5, R 1 =C 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com