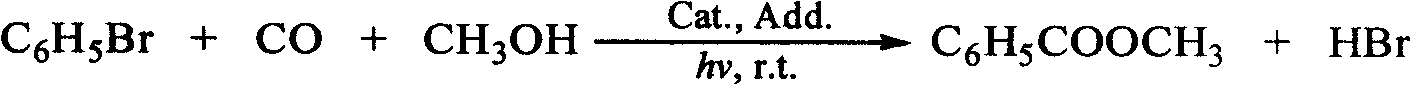Method for synthesis of niobe oil by catalyzing bromobenzene with cobalt salt in light acceleration
A methyl benzoate and light-promoted technology, which is applied in the direction of carbon monoxide or formate reaction preparation, organic chemistry, etc., can solve the problems of poor selectivity and low yield of methyl benzoate, and achieve high added value and high yield. High efficiency and selectivity, easy to store and use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Place a 400W high-pressure mercury lamp inside a hollow interlayer quartz photoreactor, pass circulating water into the interlayer, and put the entire reactor into a water tank filled with water to cool the light source; will contain 100mmol / L bromobenzene, 10mmol / L Catalyst Co(OAc) 2 , 200mmol / L alkaline additive NaOCH 3 Add 5mL of methanol solution into the quartz test tube, put the quartz test tube close to the outer wall of the photoreactor, pass carbon monoxide into the reaction solution, replace the deoxygenation, and keep the constant pressure at 0.1MPa at room temperature, start the light source to irradiate the reaction solution for 5h, the obtained product is Decane was used as internal standard, and the product was analyzed by GC and GC-MS. The yield of methyl benzoate calculated by GC was 47%, and the selectivity was 47%.
Embodiment 2
[0020] Place a 400W high-pressure mercury lamp inside a hollow interlayer quartz photoreactor, pass circulating water into the interlayer, and put the entire reactor into a water tank filled with water to cool the light source; will contain 100mmol / L bromobenzene, 10mmol / L Catalyst Co(OAc) 2 , 200mmol / L alkaline additive NaOCH 3 Add 5mL of methanol solution of 340mmol / L acetophenone into the quartz test tube, put the quartz test tube close to the outer wall of the photoreactor, pass carbon monoxide into the reaction solution, replace and remove oxygen, and keep the pressure at room temperature at a constant pressure of 0.1MPa to start the light source irradiation reaction In solution 5h, the obtained product was analyzed by GC and GC-MS with n-decane as internal standard. The yield of methyl benzoate calculated by GC was 91%, and the selectivity was 91%.
Embodiment 3
[0022] Place a 400W high-pressure mercury lamp inside a hollow interlayer quartz photoreactor, pass circulating water into the interlayer, and put the entire reactor into a water tank filled with water to cool the light source; will contain 100mmol / L bromobenzene, 10mmol / L Catalyst Co(OAc) 2 , 900mmol / L alkaline additive NaOAc and 5mL methanol solution of 340mmol / L acetophenone were added to the quartz test tube, and the quartz test tube was placed close to the outer wall of the photoreactor. The pressure was kept constant at 0.1 MPa, and the light source was turned on to irradiate the reaction solution for 5 hours. The obtained product was analyzed by GC and GC-MS with n-decane as internal standard. The yield of methyl benzoate calculated by GC was 72%, and the selectivity was 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com