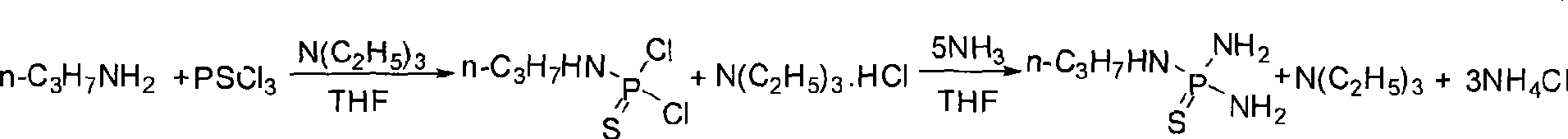Preparation of N-alkyl thiophosphoryl triamide by one-pot method
A technology of hydrocarbyl thiophosphoryl triamine and hydrocarbyl thiophosphoryl dichloride, which is applied in the field of chemical synthesis, can solve the problems of long working hours, many reaction steps and high cost, and achieves a simple process, suitable reaction conditions and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1 Preparation of N-n-propylthiophosphoric triamide
[0020] One-pot preparation reaction equation:
[0021]
[0022] Under nitrogen protection, THF134.40g was added to a 1000ml four-neck flask equipped with mechanical stirring, a thermometer, a constant pressure titration funnel, and a reflux condenser, and phosphorus trichloride (PSCl 3 ) 51.9g; 18.3g of n-propylamine (NPA) and 30.4g of triethylamine (TEA) were mixed and dissolved in 136.0g of THF and added to a 250ml constant-pressure titration funnel; The rate of addition was completed dropwise in 2 hours and 30 minutes, and the dropwise addition was completed, and the stirring was continued for 1 hour. Install the ammonia intubation tube, open the ammonia valve to feed ammonia gas, control the ammonia pressure to less than 0.12MPa; control the temperature at 5°C, pass ammonia gas for 2 hours, and at the same time the system will no longer absorb ammonia gas, slowly warm up to room temperature, stop S...
Embodiment 2
[0023] Embodiment 2 Preparation of N-n-butylthiophosphoric triamide
[0024] The one-pot preparation reaction equation is as follows:
[0025]
[0026] Under nitrogen protection, THF158.6g was added to a 1000ml four-necked flask equipped with mechanical stirring, a thermometer, a constant pressure titration funnel, and a reflux condenser, and phosphorus trichloride (PSCl 3 ) 50.9g; 22.6g of n-butylamine (NBA) and 30.4g of triethylamine (TEA) were mixed and dissolved in 138.9g of THF and added to a 250ml constant pressure titration funnel; the reaction temperature was controlled to reach -5°C, and the dropwise addition , control the rate of addition, the dropwise addition is completed in 3 hours and 30 minutes, and the dropwise addition is completed, and the stirring is continued for 1 hour. Install the ammonia intubation tube, open the ammonia valve to feed ammonia gas, and control the pressure of ammonia gas to be less than 0.12MPa; control the temperature at 0°C to 5°C, ...
Embodiment 3
[0027] Embodiment 3 Preparation of N-isobutylphosphorothiotriamide
[0028] The one-pot preparation reaction equation is as follows:
[0029]
[0030] Under nitrogen protection, THF135.7g was added to a 1000ml four-necked flask equipped with mechanical stirring, a thermometer, a constant pressure titration funnel, and a reflux condenser, and phosphorus trichloride (PSCl 3 ) 51.9g; 22.6g of iso-n-butylamine and 30.4g of triethylamine (TEA) were mixed and dissolved in 136.6g of THF and added to a 250ml constant pressure titration funnel; the reaction temperature was controlled to reach 0°C, and the dropwise addition was started. Acceleration, the dropwise addition was completed in 3 hours, and the dropwise addition was completed, and the stirring was continued for 1 hour. Install the ammonia intubation tube, open the ammonia valve to feed ammonia gas, control the pressure of ammonia gas to less than 0.12MPa, control the temperature at 5°C, pass ammonia gas for 2 hours, slowly ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com