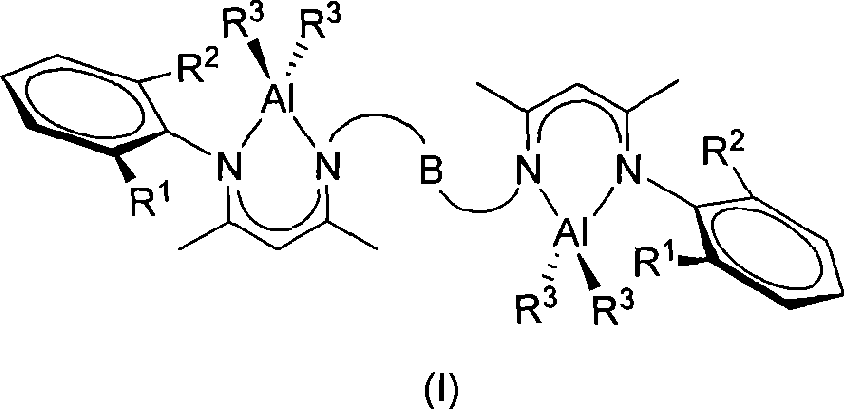
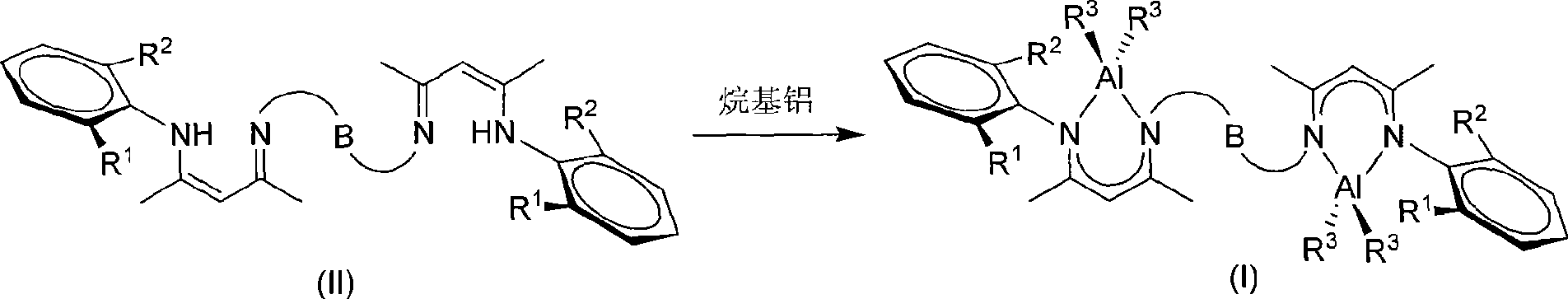
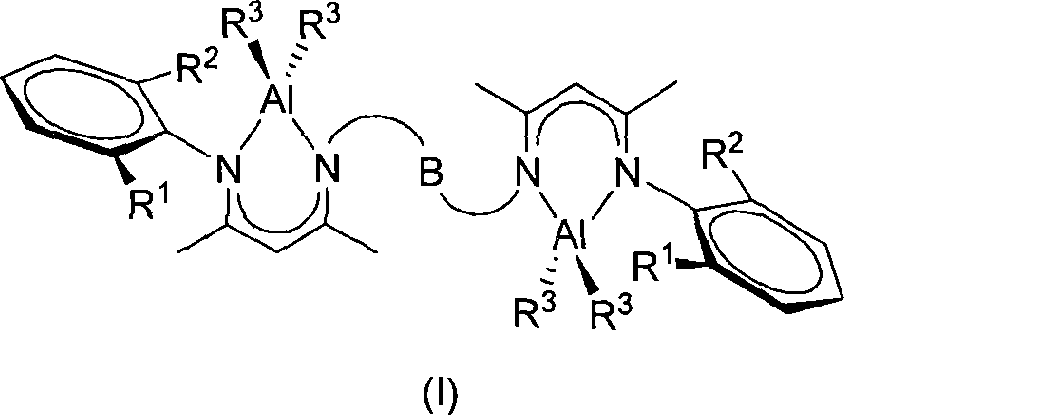
Novel bridged beta-diimido binuclear aluminum compound and preparation method and use thereof
A technology of aluminum compounds and diimines, which is applied in the field of new bridged β-diimine binuclear aluminum compounds, can solve problems such as aluminum complexes containing bridged β-diimine ligands that have not been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Under argon protection, add 17.16g (50mmol) 4-(2-trifluoromethylphenyl)amino-3-penten-2-one to a 150mL Schlenk bottle, 20mL CH 2 Cl 2 , 9.501g (50mmol) [Et 3 O]+[BF4 ] - 10mLCH 2 Cl 2 Solution, after the dropwise addition, return to room temperature and stir, then add dropwise 15.15g (150mmol) triethylamine and 1.851g (25mmol) 1,3-propanediamine mixed solution to the above reaction solution, and stir for 12 hours after the dropwise addition, then reduce Remove the solvent under pressure to obtain a yellow sticky solid, add 80mL of toluene, filter and drain the solvent to obtain a yellow solid, the initial product is recrystallized with toluene to obtain a light yellow bridged β-diimine ligand: 1,3- Bis(2-(2-trifluoromethylanilino)-pent-2-en-4-ketimino)propane (8.122 g, 62% yield). Its structure is as follows:
[0032]
Embodiment 2
[0034] Under argon protection, add the bridged β-diimine ligand 1,2-bis(2-(2,6-diisopropylanilino)-pent-2-ene-4- Ketoimino) ethane (0.457g, 4.000mmol), n-hexane 30mL, drop AlEt therein at 0°C 3 (0.457g, 4.000mmol), after the dropwise addition was completed, it was allowed to rise to room temperature and stirred, reacted for 12 hours, filtered, concentrated and placed at -20°C to obtain colorless crystals (0.898g, 75%). Code 1a. Its structure is as follows:
[0035]
[0036] 1 H NMR (500MHz, CDCl 3 , 25°C): δ-0.30(dq, 2 J=14.3Hz, 3 J=8.2Hz, 4H, AlCH 2 CH 3 ), -0.10(dq, 2 J=14.3Hz, 3 J=8.2Hz, 4H, AlCH 2 CH 3 ), 0.91(t, 3 J=8.2Hz, 12H, AlCH 2 CH 3 ), 1.22(d, 3 J=6.8Hz, 12H, CH(CH 3 ) 2 ), 1.30(d, 3 J=6.8Hz, 12H, CH(CH 3 ) 2 ), 1.78 (s, 6H, CH 3 ), 2.28(s, 6H, CH 3 ), 3.09(q, 3 J=6.8Hz, 4H, CH(CH 3 ) 2 ), 3.52 (s, 4H, N-CH 2 ), 5.05(s, 2H, γ-CH), 7.24(t, 3 J=7.4Hz, 2H, p-Ar-H), 7.31(d, 3 J=7.4Hz, 4H, m-Ar-H). 13 C{ 1 H} NMR (100MHz, CDCl 3 , 25°C):...
Embodiment 3
[0038] Under argon protection, add ligand 1,2-bis(2-(2,6-dimethylanilino)-pent-2-en-4-ketimino)ethane ( 1.085g, 2.000mmol), n-hexane 30mL, drop AlEt therein at 0°C 3 (0.457g, 4.000mmol), after the dropwise addition, let it rise to room temperature and stir naturally, react for 12 hours, then filter, concentrate and put at -20°C to obtain colorless crystals (1.023g, 72%). Code 1b. Its structure is as follows:
[0039]
[0040] 1 H NMR (500MHz, CDCl 3 , 25°C): δ-0.27(dq, 2 J=14.3Hz, 3 J=8.1Hz, 4H, AlCH 2 CH 3 ), -0.16(dq, 2 J=14.3Hz, 3 J=8.1Hz, 4H, AlCH 2 CH 3 ), 0.75(t, 3 J=8.1Hz, 12H, AlCH 2 CH 3 ), 1.59 (s, 6H, CH 3 ), 2.14(s, 12H, Ar-CH 3 ), 2.17(s, 6H, CH 3 ), 3.44(s, 4H, N-CH 2 ), 4.85(s, 2H, γ-H), 7.04(m, 6H, Ar-H). 13 C{ 1 H} NMR (100MHz, CDCl 3 , 25°C): δ0.9(AlCH 2 CH 3 ), 9.5 (AlCH 2 CH 3 ), 18.7 (Ar-Me), 22.1 (CMe), 22.7 (CMe), 48.6 (N-CH 2 ), 98.0(CH), 125.9(Ar-C), 128.7(Ar-C), 134.0(Ar-C), 144.1(Ar-C), 168.1(NCMe), 170.1(NCMe).Anal.Calcd....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conversion rate | aaaaa | aaaaa |
| Conversion rate | aaaaa | aaaaa |
| Conversion rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com