Use of ligustrazine phosphate in preparing medicine for treating dilated cardiomyopathy
A technology for dilated cardiomyopathy and ligustrazine phosphate, applied in the field of medicine, can solve problems such as unreported, achieve strong scientific and innovative effects, improve the structure of intercalated discs, and reduce mitochondrial swelling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Effect of long-term administration of ligustrazine phosphate on the survival rate of model mice with dilated heart disease:
[0019] 1. Animal grouping and administration: identification of cTnT by PCR R141W Transgenic positive mice. The negative mice were used as controls, and the positive mice were evenly divided into a model group and a ligustrazine phosphate administration group according to the results of echocardiography 1.5 months after birth, with 12 animals in each group. Tetramethylpyrazine phosphate (45 mg / kg, approved by the Chinese Medicine H11021964, Beijing Yanjing Pharmaceutical Co., Ltd.) was administered orally for 6 months.
[0020] 2. Survival Analysis: The cTnT R141W In the transgenic dilated cardiomyopathy model, individuals died during the breeding process. We recorded the number of animals that died in each group from the beginning of administration to the end of observation. The number of animals that died in the negative control group was 0, ...
Embodiment 2
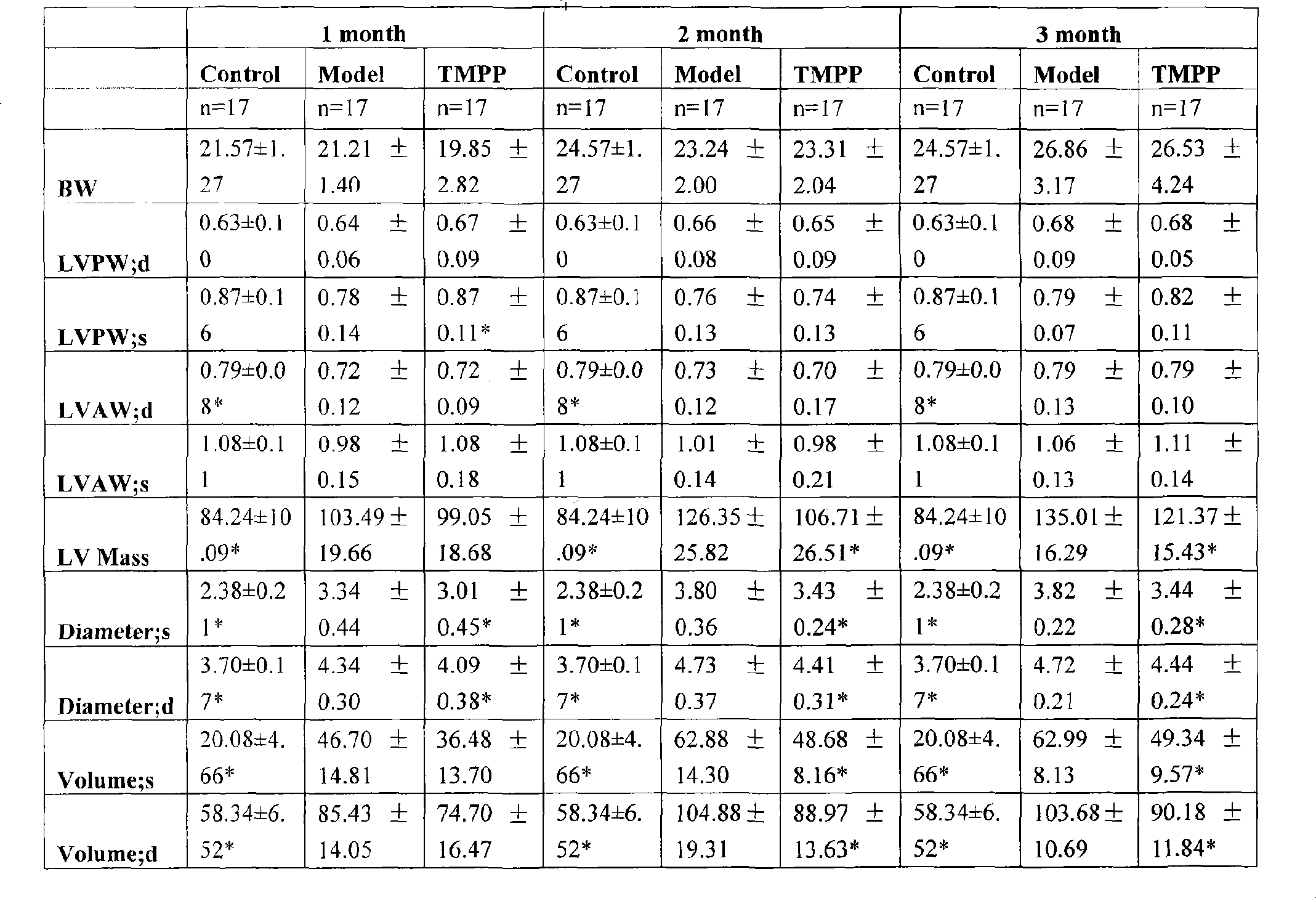
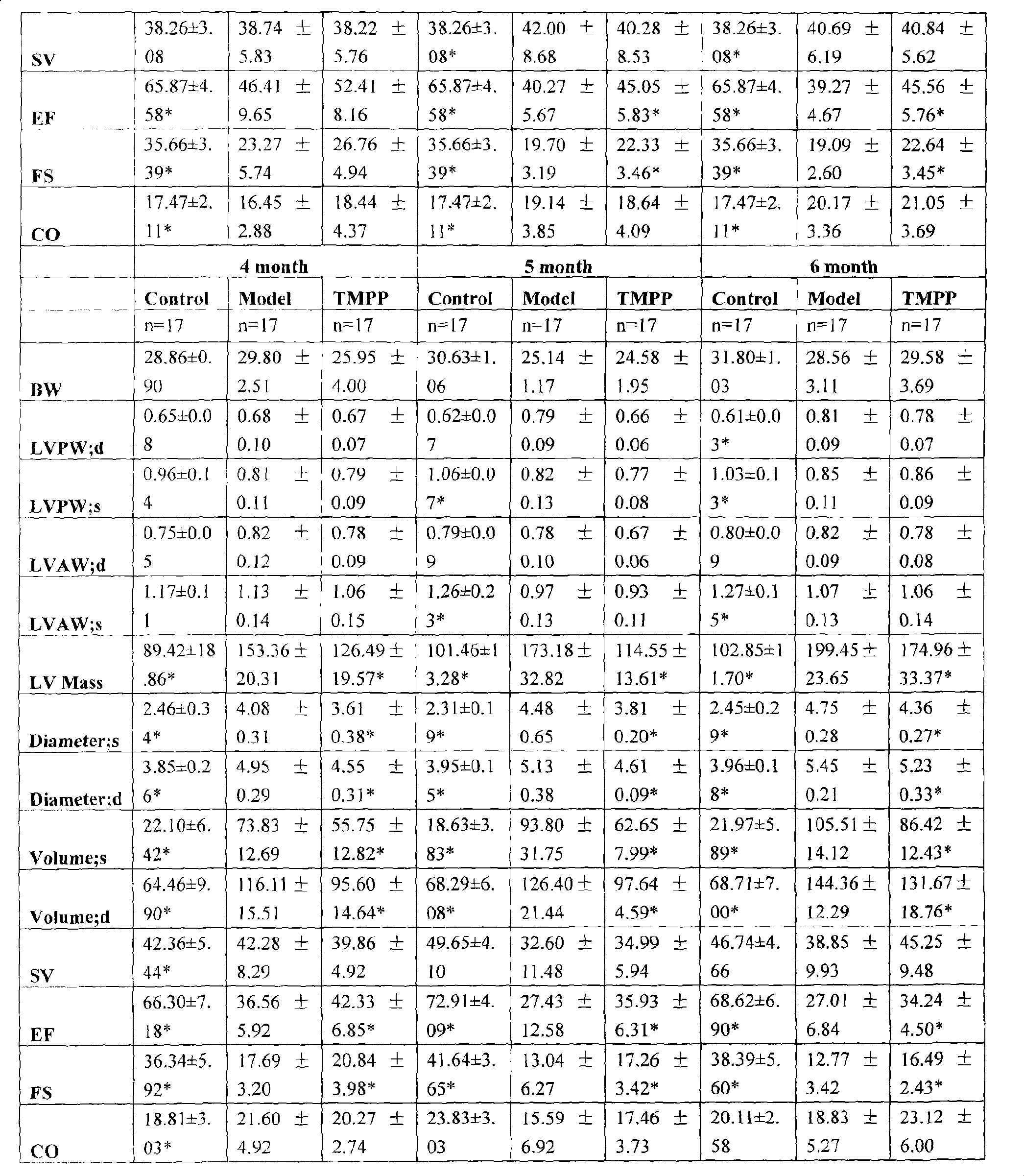
[0022] Effects of long-term administration of ligustrazine phosphate on cardiac function and configuration of model mice with dilated heart disease (ultrasound):
[0023] Echocardiography was performed once a month, under tribromoethanol anesthesia, and a small animal ultrasound instrument (Vevo770 small animal ultrasound detection system, Canada) was used to scan the probe at 30 MHz. The long-axis M-ultrasound results of the left ventricle were analyzed by echocardiography. Heart shape and heart function of animals in each group, including BW (body weight, body weight), LVPW; d (thickness of left ventricular posterior wall in diastole), LVPW; s (thickness of left ventricular posterior wall in systole), LVAW; d (diastolic left ventricular anterior wall thickness), LVAW; s (systolic left ventricular anterior wall thickness), LV Mass(AW) (left ventricular weight), Diameter; s (systolic left ventricular diameter), Diameter; d( Diastolic left ventricular diameter), Volume; s (syst...
Embodiment 3
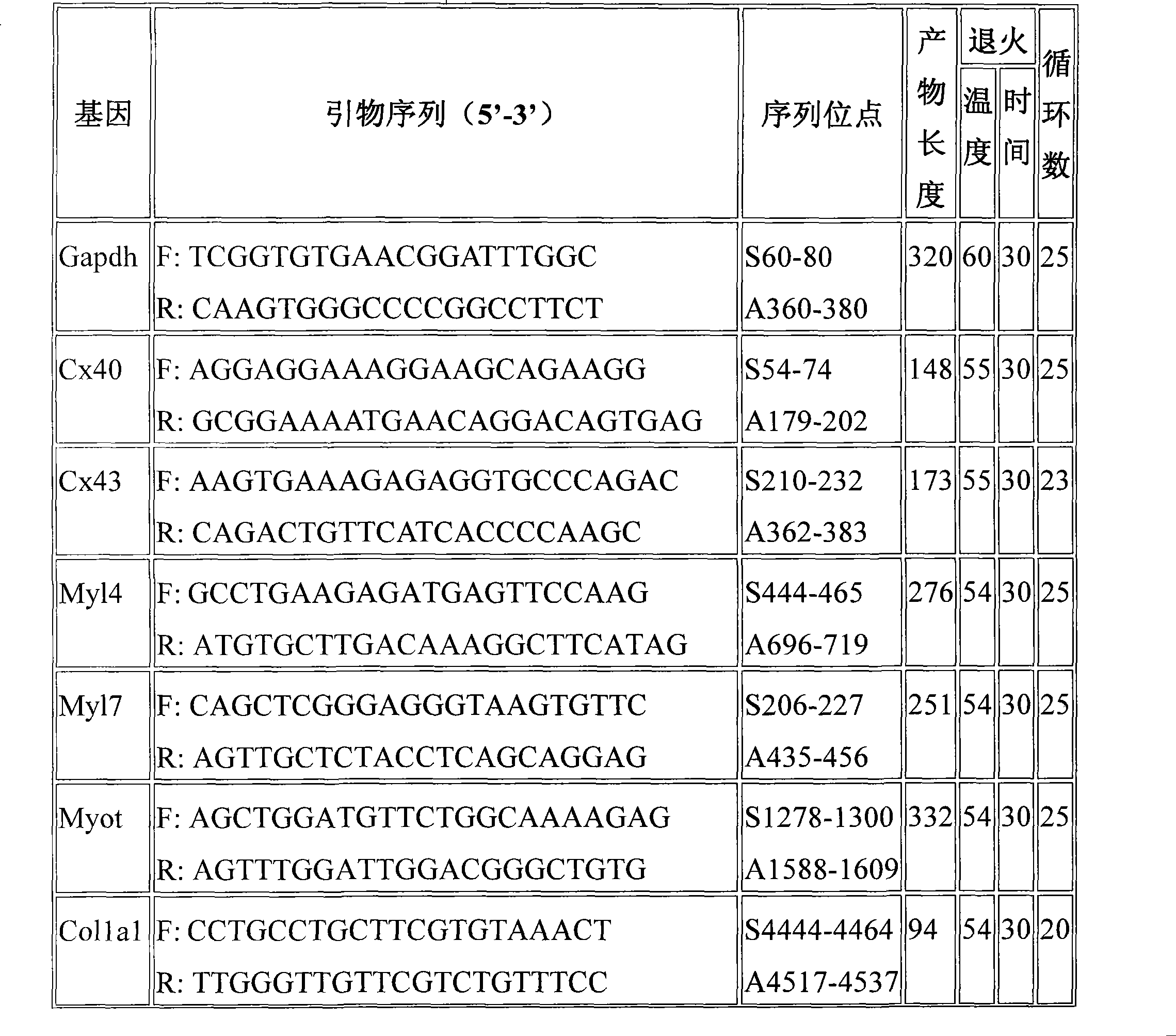
[0028] Effects of Long-term Administration of Ligustrazine Phosphate on the Microstructure of the Heart in Mice with Dilated Cardiac Disease
[0029] (HE and Masson staining):
[0030] 1. Method: fix the heart, paraffin section, conventional HE staining. Masson composite staining solution for 5 minutes, 0.2% acetic acid aqueous solution for a little washing, 5% phosphotungstic acid for 5-10 minutes, 0.2% acetic acid aqueous solution for 5 minutes, bright green staining solution for 5 minutes. Immerse twice in 0.2% acetic acid aqueous solution, dehydrate with absolute ethanol, make xylene transparent, and seal with neutral gum. Observation under a light microscope: collagen fibers are green, muscle fibers are red, and red blood cells are orange.
[0031] 2. Results: Observed under the light microscope, the myocardial fibers in the model group were unevenly hypertrophied, arranged in disorder, and some cells were vacuolated; the nucleus of the myocardial cells was large, deepl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com