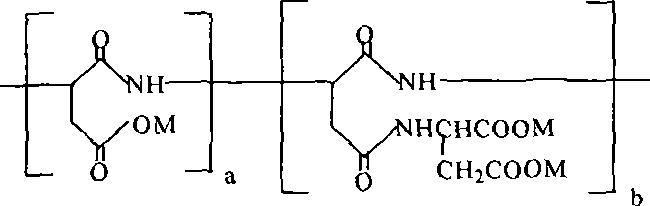
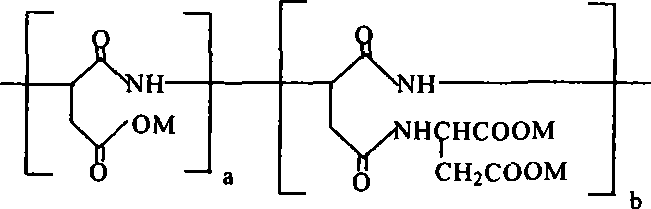
Biodegradable scale inhibitor-carboxylic acid base-containing poly-asparagic acid derivant and method for producing the same
A corrosion and scale inhibitor, aspartic acid technology, applied in chemical instruments and methods, scale removal and water softening, water/sludge/sewage treatment, etc., to achieve good scale inhibition effect, good biodegradability and slow down. Corrosion performance, improve the effect of scale inhibition and dispersibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Take 300 grams of aspartic acid and crush it in a mortar. After passing through a 180-mesh sieve, spread it on a medical tray. Then put the tray into an electric blast drying box for polycondensation reaction. The reaction temperature is 240°C and the reaction time is 4 hours. Stir once for 1 hour to obtain 217 grams of yellow-brown polysuccinimide solid; Dissolve 13.3 grams of aspartic acid in water and add 17.6 grams of 25% sodium hydroxide aqueous solution dropwise with stirring, and control the reaction temperature to 20-50°C ; Add 9.7 grams of polysuccinimide to a four-necked flask equipped with a stirring, thermometer and dropping funnel, then add 30 mL of water to suspend it in the water, and slowly add dropwise sodium hydrogen aspartate solution under stirring, Adjust the pH to 8 with 25% sodium hydroxide aqueous solution every half an hour and react for 24 hours to obtain a reddish-brown polyaspartic acid derivative aqueous solution. Adjust the pH to 2~3 with hydro...
Embodiment 2
[0026] Take 300 grams of aspartic acid and crush it in a mortar, spread it through a 180-mesh sieve, and spread it on a medical tray. Then put the tray into an electric blast drying box for polycondensation reaction. The reaction temperature is 230 ℃, and the reaction time is 5 hours. Stir once for 1 hour to obtain 217 grams of yellow-brown polysuccinimide solid; dissolve 15.96 grams of aspartic acid in water and add 23.04 grams of 25% sodium hydroxide aqueous solution dropwise under stirring, and control the reaction temperature to 20-50°C ; Add 9.7 grams of polysuccinimide to a four-necked flask equipped with a stirring, thermometer and dropping funnel, then add 30 mL of water to suspend it in the water, and slowly add dropwise sodium hydrogen aspartate solution under stirring, Adjust the pH to 9 with 25% sodium hydroxide aqueous solution every half hour, react for 24 hours to obtain a reddish-brown polyaspartic acid derivative aqueous solution, adjust the pH to 2 to 3 with hydr...
Embodiment 3
[0028] Take 300 grams of aspartic acid and crush it in a mortar. After passing through a 180-mesh sieve, spread it on a medical tray. Then put the tray into an electric blast drying oven for polycondensation reaction. The reaction temperature is 250°C and the reaction time is 3.5 hours. Stir once every 1 hour to obtain 217 grams of yellow-brown polysuccinimide solid; dissolve 15.96 grams of aspartic acid in water and add 24.96 grams of 25% sodium hydroxide aqueous solution dropwise under stirring, and control the reaction temperature to 20-50 ℃; Add 9.7 grams of polysuccinimide into a four-necked flask equipped with a stirring, thermometer and dropping funnel, then add 30 mL of water to suspend it in the water, and slowly add sodium hydrogen aspartate solution dropwise under stirring , Adjust the pH to 10 with 25% sodium hydroxide aqueous solution every half an hour, react for 24 hours to obtain a reddish-brown polyaspartic acid derivative aqueous solution, adjust the pH to 2-3 wi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| scale inhibition rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com