Process for producing dicyclohexyl methyl hydride diisocyanate and its midbody
A technology of dicyclohexylmethane and diaminodicyclohexylmethane, which is applied in the field of preparation of diisocyanate and its intermediates, can solve the problems of shortened catalyst life, increased production cost, and reduced catalyst activity, and achieve extended use Effects of longevity, reduction in toxicity, and reduction in production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
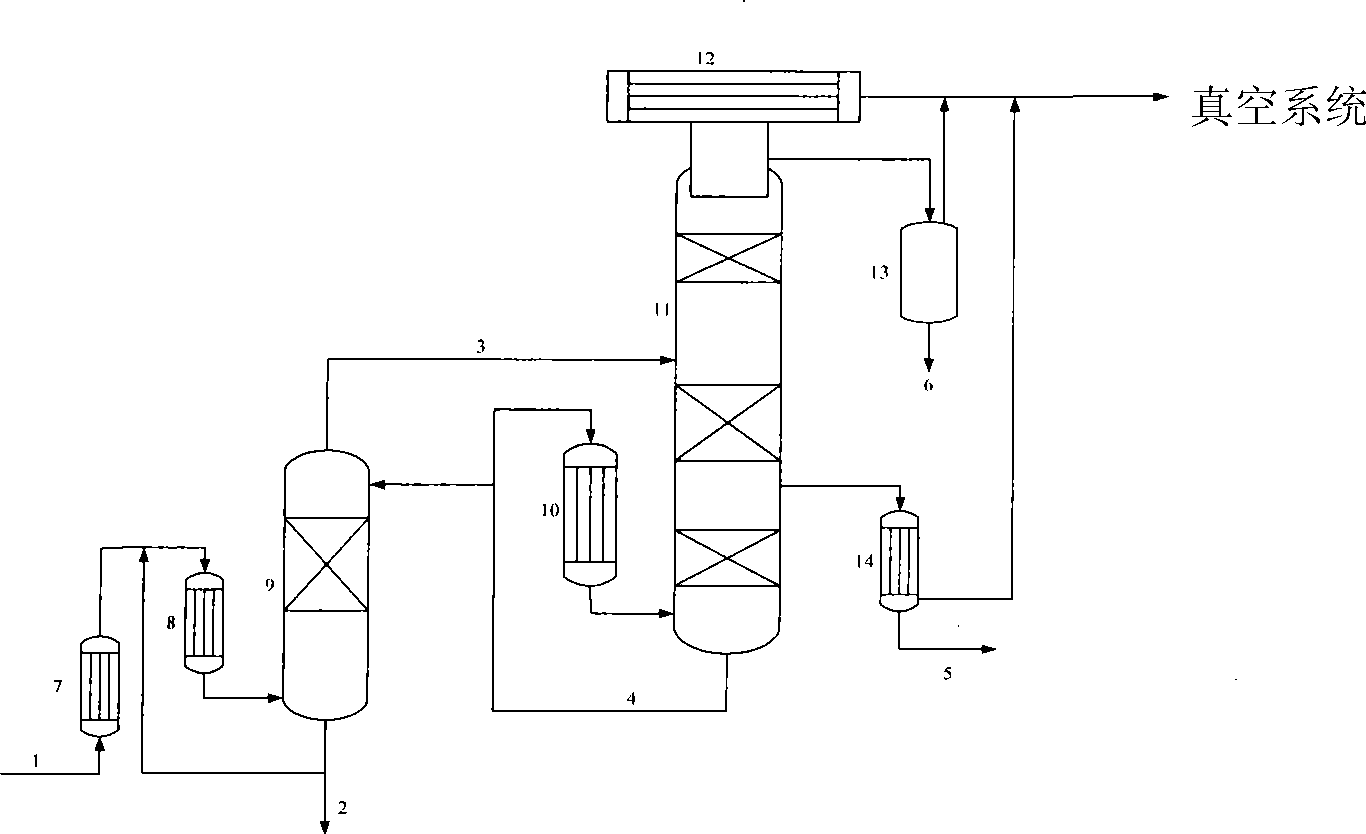
[0055] use figure 1In the process shown, the bicyclic MDA and polycyclic MDA from the condensation process of the diphenylmethane diisocyanate (MDI) plant are fully preheated to 220°C by the preheater and the falling film evaporator, and then enter the distillation tower with a diameter of 50mm , the column is filled with corrugated structured packing, the number of trays is 5, the bottom temperature is 250°C, the top temperature is 230°C and the top pressure is 3.5 mbar; the feed composition is 4,4'-diaminodiphenyl The methane content is 68wt%, the 2,4'-diaminodiphenylmethane content is 5wt%, and the polycyclic MDA is about 27wt%. The vapor phase material at the top of the distillation tower enters the middle of the rectification tower. The temperature at the bottom of the rectification tower is 244°C, the temperature at the top of the tower is 190°C, the temperature of the side line gas phase in the tower is 240°C, and the number of trays in the rectification tower is 25. ...
Embodiment 2
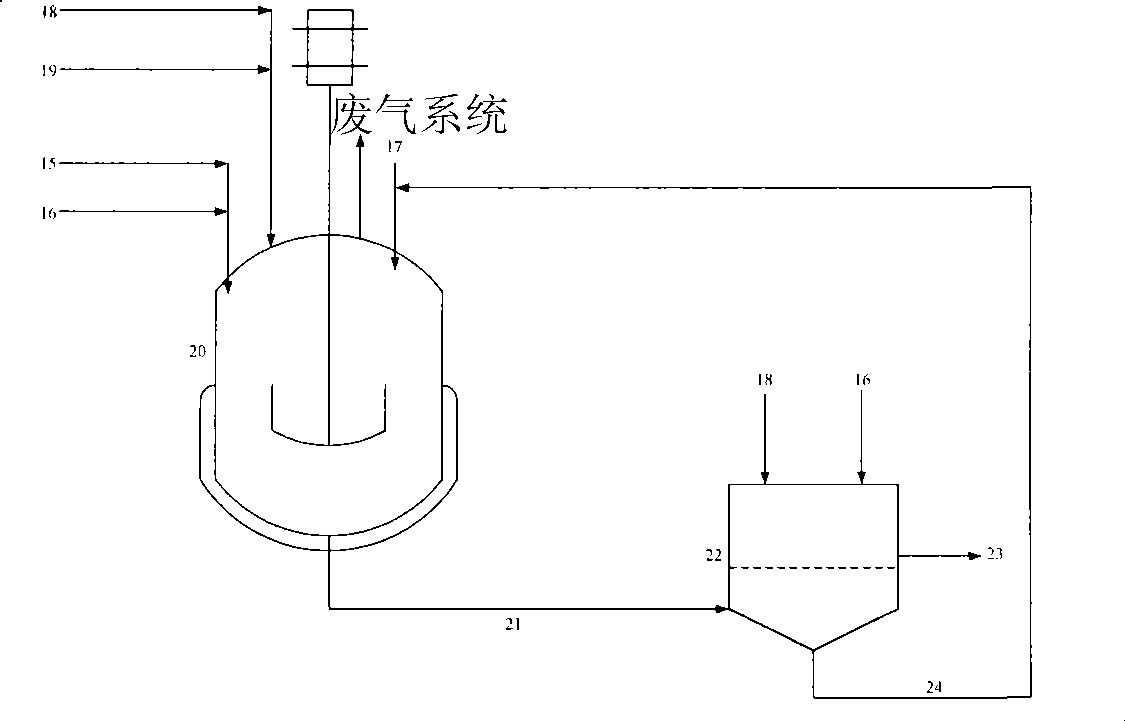
[0059] use figure 2 In the process shown, 4,4'-diaminodiphenylmethane content of 99.7wt% diaminodiphenylmethane and tetrahydrofuran are added to the autoclave at a mass ratio of 1:1, and 3wt% Rh / Al 2 o 3 The catalyst is added into the kettle, and the amount of the catalyst added accounts for 1.2wt% of the feeding amount of diaminodiphenylmethane. After replacing the reactor with nitrogen and hydrogen, fill the reactor with hydrogen to 5MPa, raise the temperature of the material in the reactor to 185°C, maintain the temperature, and maintain the hydrogen pressure in the reactor at 5MPa for 240 minutes. Cool down to 40°C and replace with pressure release, filter the reaction liquid in the kettle, flush the catalyst with trapped solids into the reaction kettle through backwashing operation and continue to be used for the hydrogenation reaction of the next batch, the main component of the filtrate It is a solution of diaminodicyclohexylmethane in tetrahydrofuran. The filtrat...
Embodiment 3
[0061] use figure 2 In the process shown, the diaminodiphenylmethane isomer mixture with a 4,4'-diaminodiphenylmethane content of 50.12wt% and cyclohexylamine are added to the high-pressure stirred tank at a mass ratio of 3:10. 5wt% Ru / C catalyst was added into the kettle, and the amount of catalyst added accounted for 5wt% of the feeding amount of diaminodiphenylmethane. After replacing the reactor with nitrogen and hydrogen, fill the reactor with hydrogen to 9MPa, raise the temperature of the material in the reactor to 180°C, maintain the temperature, and maintain the hydrogen pressure in the reactor at 9MPa for 360 minutes. After cooling and filtering, the filtrate sample was taken for gas chromatography analysis, and the content of diaminodiphenylmethane was 2.36wt%, and the content of diaminodicyclohexylmethane was 91.75wt%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com