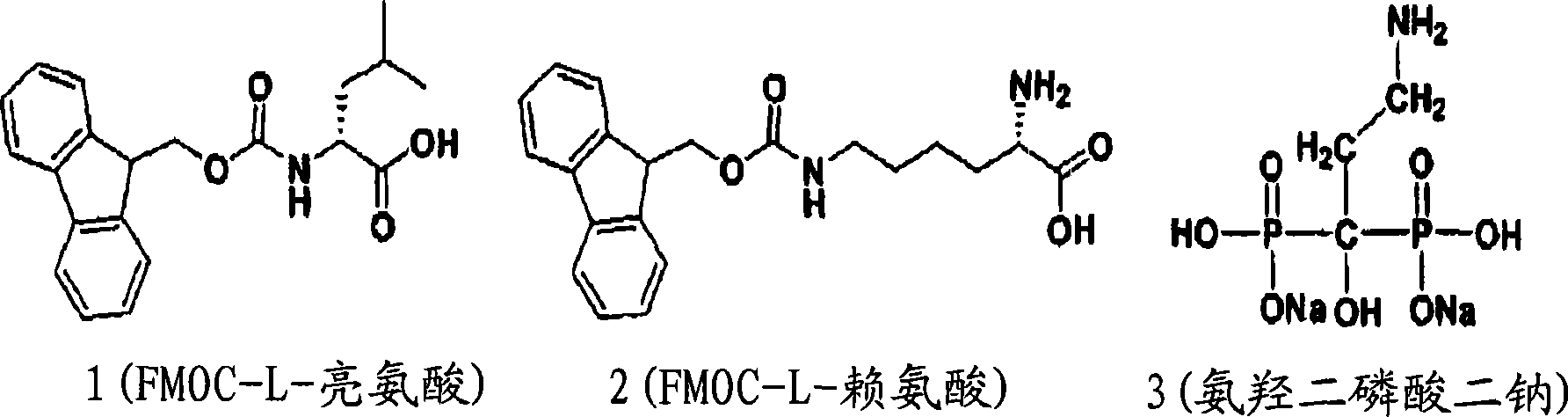
Multifunctional supramolecular hydrogels as biomaterials
A technology of supramolecular hydrogels and functional molecules, applied in the field of multifunctional supramolecular hydrogels as biological materials, can solve the problems of single function and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
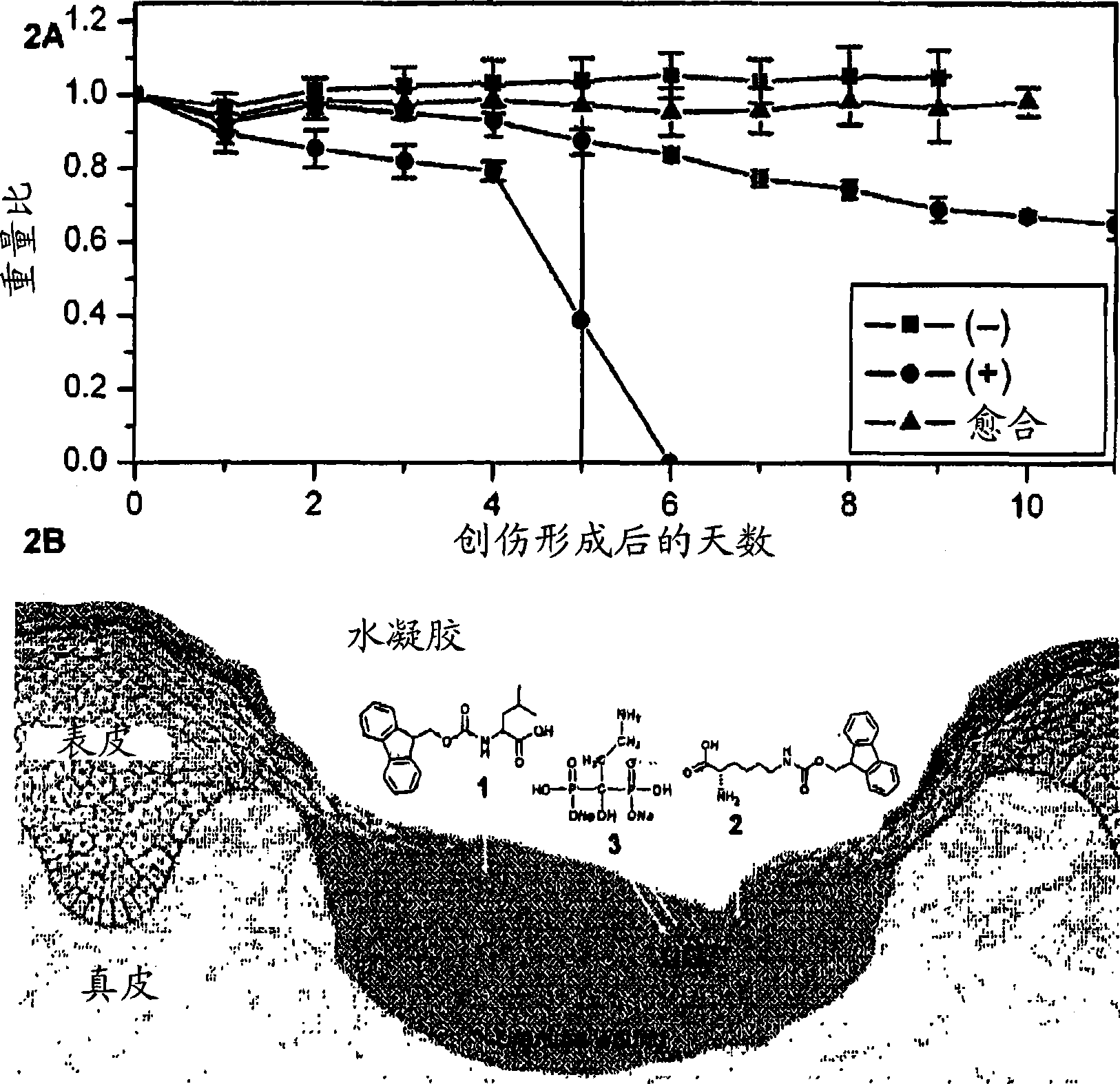
[0048] Example 1. Wound Healing
[0049] In order to illustrate the biological activity of the supramolecular hydrogel of the present invention, it contains such as figure 1 Hydrogels of the functional molecules shown were used to treat uranium wounds by abrading the skin on the backs of mice and administering uranyl nitrate superficially to the wounds. Then, the hydrogel was then topically administered for 20 minutes on the wound of the negative control group, but not the positive control group. The result of the experiment is as figure 2 As shown in A. Mice in all groups showed a loss of original body weight on the second day due to the effect of trauma. The negative control group healed the wound quickly after experiencing a slight loss of original body weight and returned to normal growth the next day. In contrast, the positive control group exhibited sustained weight loss until death at about day 5 or a 35% weight loss over the next ten days. Thus, when the hydrog...
Embodiment 2
[0052] Example 2. Non-covalently cross-linked supramolecular hydrogels
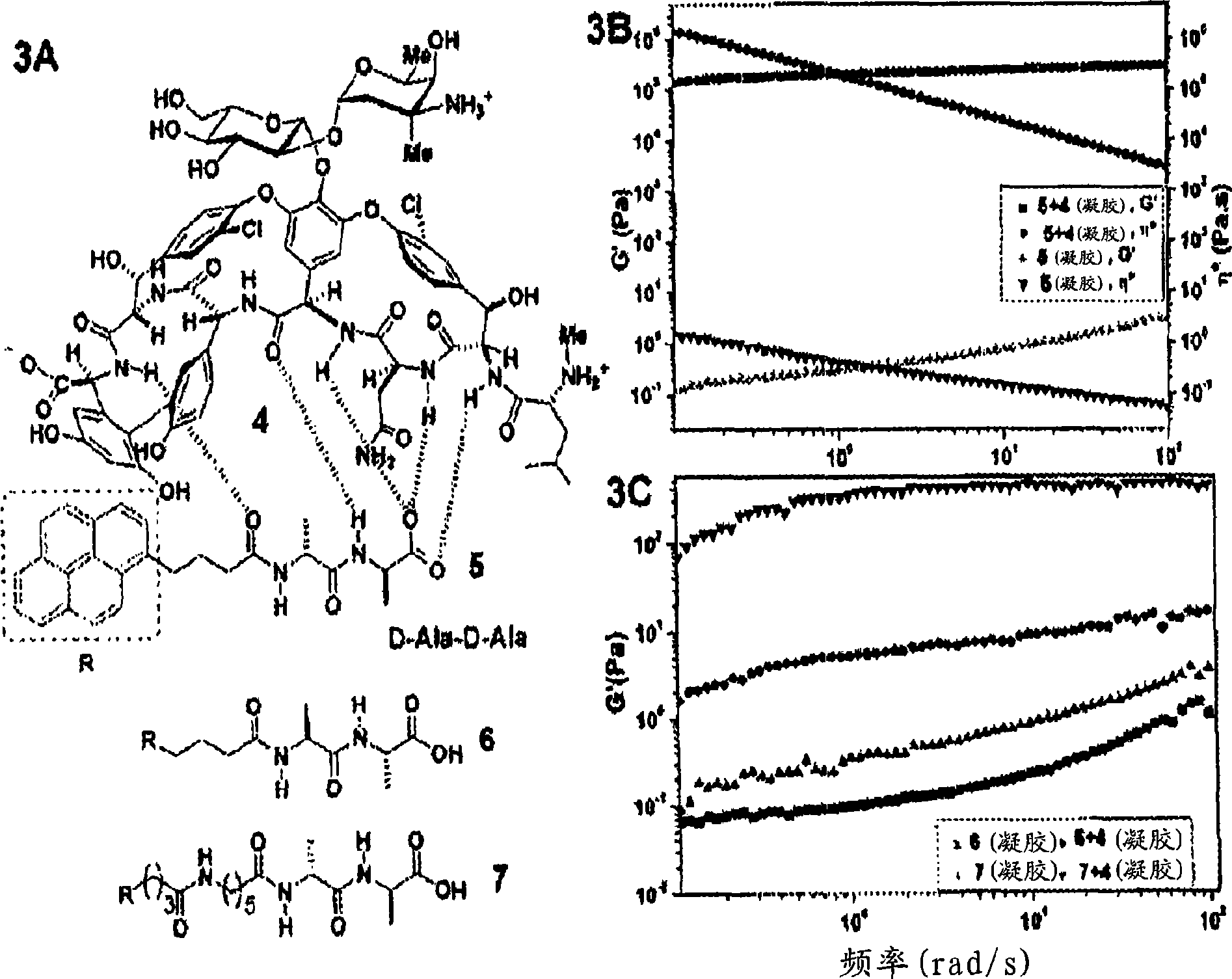
[0053] While in situ polymerization allows for enhanced stability of small molecule gels, such methods of covalent crosslinking typically require additional chemical synthesis, which alters the properties of the hydrogelator and may lead to loss of biocompatibility and Biodegradability. Therefore, it is preferable to use molecular recognition (non-covalent cross-linking) to enhance the elasticity of small molecule hydrogels. For example, addition of ligands to mechanically poor hydrogels of receptor derivatives resulted in up to a million-fold increase in the hydrogel storage modulus. The term "non-covalent crosslinking" means that the crosslinking is achieved by hydrogen bonding, hydrophobic forces or ionic forces.
[0054] In one embodiment, vancomycin (Van) was chosen as ligand 4 and D-Ala-D-Ala derivative was chosen as receptor 5 because of the well established molecules between 4 and 5 in aqueous...
Embodiment 3
[0057] Example 3. Antibiotic Supramolecular Hydrogel
[0058] Figure 4A The chemical structure of 8 is shown (when R = pyrenyl), Figure 4B A photograph showing the formation of a hydrogel by adding 6.5 mg of 8 to 1.8 ml of water corresponds to ~0.36 wt% (2.2 mM) gelator and ~23000 water molecules / gelator molecule. 8 Unexpectedly potent (0.125-2μg / ml, 8-11 times lower dilution than the corresponding vancomycin), inhibiting VRE (2 vanA-positive Enterococcus faecalis, 4 vanA-positive Streptococcus faecalis, 4 vanB-positive Enterococci). The strong propensity and unexpected potency of 8 for self-assembly also led us to speculate that 8 may aggregate into supramolecular structures at the cell surface when its local concentration is high.
PUM
| Property | Measurement | Unit |
|---|---|---|
| storage modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com