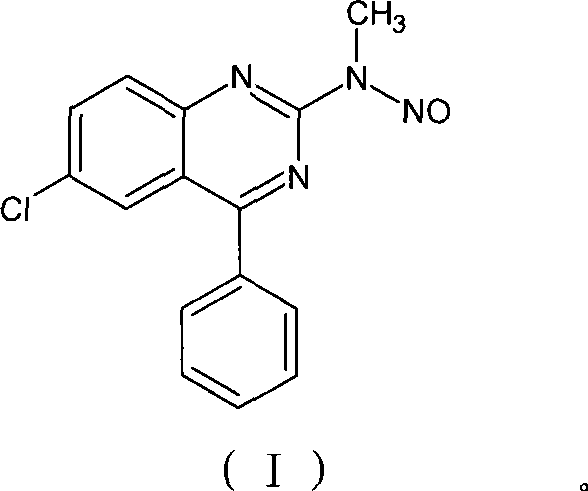
Benzodiazepinic amine nitrite compound, preparation and use thereof
A technology of benzodiazepine and compound, which is applied in the application field of preparing alprazolam, can solve the problems of environmental pollution, harsh reaction conditions, unfavorable industrial scale production, etc., and achieve the reduction of environmental pollution, reduction of reaction steps, and simple reaction easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
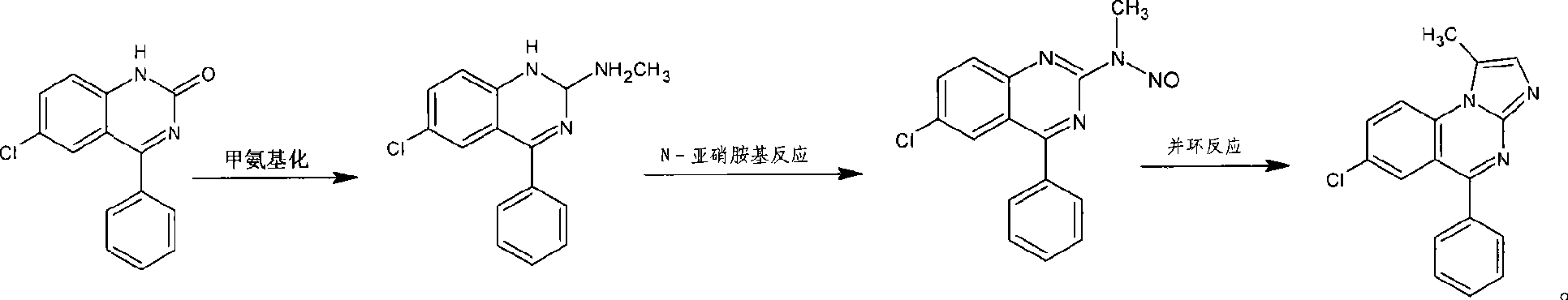
[0032] Example 1.1,4-benzodiazepine —Preparation of N-nitrosamines intermediate (I)
[0033] 1. Methylamination reaction
[0034] Into a clean flask were added 17.5 g (0.068 mol) of 7-chloro-5-phenylbenzodiazepinone (II), 90 ml of tetrahydrofuran, and 25 ml of toluene. Cool in an ice bath to 0-5°C. Methylamine gas was introduced to saturation. Then, a solution of 19 g (0.1 mol) of titanium tetrachloride and 20 ml of benzene was added dropwise to the reaction liquid over 15 minutes. After dropping, stir and heat to reflux, and react for 3 hours. After cooling to room temperature, 80 ml of water was slowly added to the solution. The insoluble matter was removed by filtration, and the filter cake was washed with a small amount of tetrahydrofuran. Combine the filtrate and washing liquid, let stand to separate layers, wash the separated organic layer with saturated sodium chloride, distill under reduced pressure to recover the solvent, add isopropanol, and precipitate a soli...
Embodiment 2
[0037] Example 2. Synthesis of Alprazolam
[0038] 1,4-benzodiazepine obtained by embodiment 1 ——Add 18g (0.06mol) of N-nitrosamine intermediate (I) to 80ml N,N-dimethylformamide, stir evenly, add 5.3g (0.0308mol) of p-toluenesulfonic acid, 5g of acetylhydrazine (0.068mol); heated to 100°C for 2 hours. After the completion of the reaction was monitored by HPLC, the temperature was lowered to room temperature, and 50 ml of saturated sodium bicarbonate solution was added to the reaction solution. Then 120ml of dichloromethane was added for extraction, and the aqueous phase was extracted with 60ml of dichloromethane. The obtained dichloromethane layers were combined and washed with water, saturated sodium bicarbonate solution, and saturated sodium chloride solution. Concentrate under reduced pressure, then add 80ml of ethanol to dilute, cool down to 0-5°C and let stand for 1 hour, then filter, wash with ethanol, and recover the mother liquor. The obtained crude product was re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com