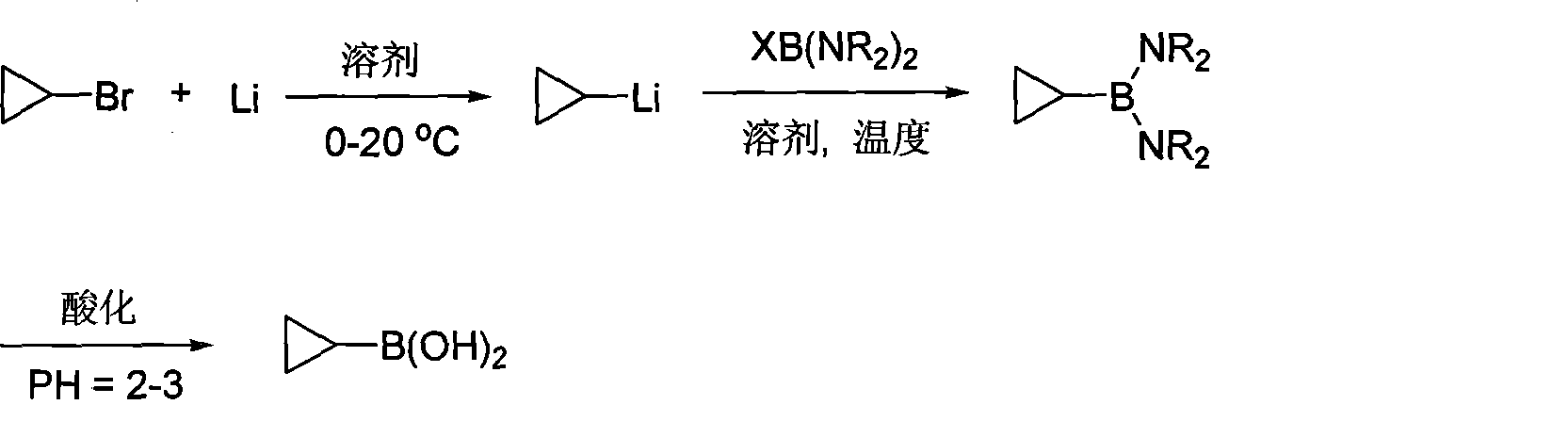
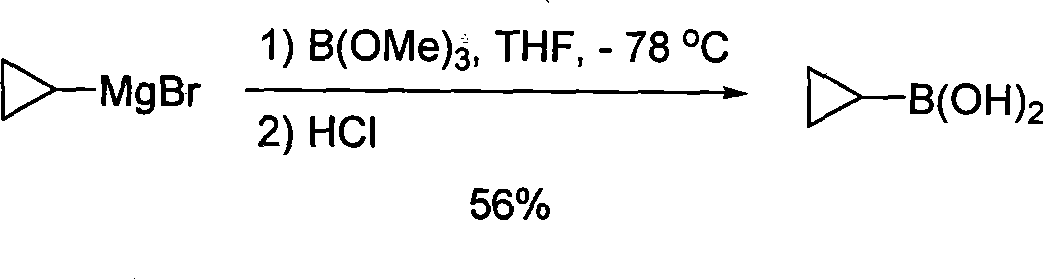Novel process for synthesizing cyclopropylboronic acid
A cyclopropylboronic acid and a new process technology are applied in the field of synthesis of organic compounds and achieve the effects of mild process conditions, easy operation, good process stability and simple synthesis steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] a) Synthesis of cyclopropyllithium: under the protection of argon, add metal lithium nails (1.1 moles), 500 ml of anhydrous tert-butyl methyl ether to a 1-liter reaction flask equipped with a condenser tube and a constant pressure addition funnel, and store at room temperature Stir for 20 minutes. Maintain the temperature at 30-40°C, start to slowly add cyclopropyl bromide (0.5 mol) dropwise, and after 4-6 hours, add cyclohexane with 1% mass of cyclopropyl bromide as internal standard analysis, keep warm and continue the reaction 3-5 hours. When the internal standard analysis of cyclopropyl bromide is less than 1%, stop the reaction. The tert-butyl methyl ether solution of cyclopropyllithium was obtained, which was directly used in the next step of synthesis under the protection of argon.
[0027] b) Synthesis of cyclopropylboronic acid: a 1-liter reaction flask under the protection of argon was cooled to about -10°C, 175 ml of cyclohexane was added, and the compound ...
Embodiment 2
[0029] a) Synthesis of cyclopropyllithium: under the protection of argon, add metal lithium nails (2.5 moles) and 500 ml of diethyl ether to a 1-liter reaction flask equipped with a condenser tube and a constant pressure addition funnel, and stir at room temperature for 20 minutes. Maintain the temperature at 0-10°C, start to slowly add cyclopropyl bromide (0.5 mol) dropwise, and after 2-3 hours, add cyclohexane with 1% mass of cyclopropyl bromide as internal standard analysis, keep warm and continue the reaction 1-2 hours. When the internal standard analysis of cyclopropyl bromide is less than 1%, stop the reaction. The diethyl ether solution of cyclopropyllithium was obtained, which was directly used in the next step of synthesis under the protection of argon.
[0030] b) Synthesis of cyclopropyl boronic acid: Cool a 1-liter reaction flask under the protection of argon to about -10°C, add 150 ml of toluene, compound BrB(NMe 2 ) 2 (0.67 mol), stirred for 30 minutes. Start...
Embodiment 3
[0032]a) Synthesis of cyclopropyllithium: under the protection of argon, add metal lithium nails (2.2 moles) and 500 ml of tetrahydrofuran to a 1-liter reaction flask equipped with a condenser tube and a constant pressure addition funnel, and stir at room temperature for 20 minutes. Keep the temperature at 20-30°C, start to slowly add cyclopropyl bromide (0.5 mol) dropwise, and after 2-5 hours, add cyclohexane with 1% mass of cyclopropyl bromide as internal standard analysis, keep warm and continue the reaction 3-5 hours. When the internal standard analysis of cyclopropyl bromide is less than 1%, stop the reaction. The tetrahydrofuran solution of cyclopropyllithium was obtained, which was directly used in the next step of synthesis under the protection of argon.
[0033] b) Synthesis of cyclopropyl boronic acid: 1 liter reaction flask under the protection of argon, cooled to about -10 ℃, add 160 ml of benzene, compound IB(NMe 2 ) 2 (0.50 mol), stirred for 30 minutes. Start...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com