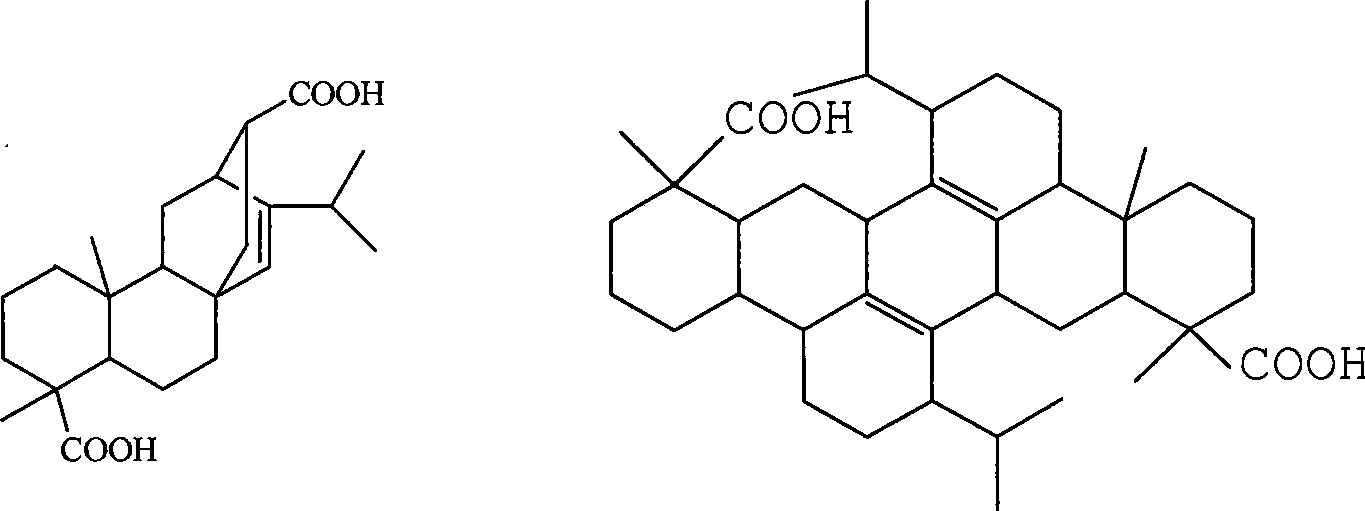
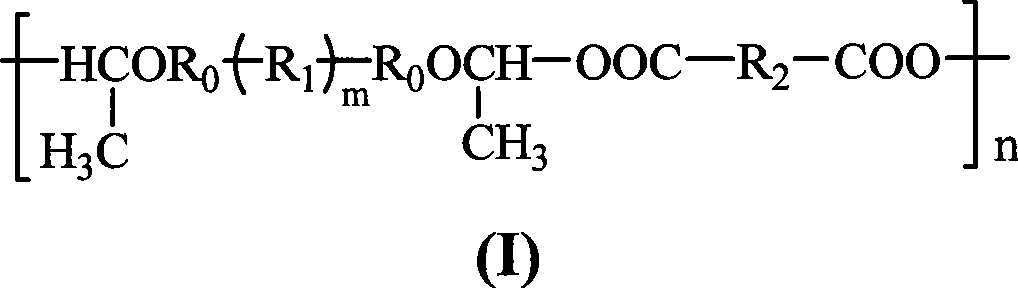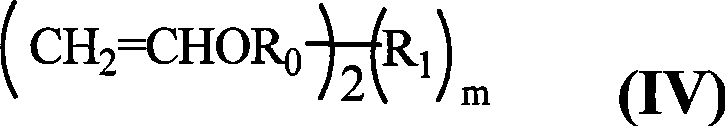Rosin diacid ester acetal polymer without aryl , process for synthesizing the same and use thereof
A technology of rosin diacid ester and acetal polymer, which is applied in the field of acetal polymer and its synthesis, and can solve the problems of increasing the difficulty of synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0039] Reference Example 2 Preparation of abietic acid dimer
[0040] Add 150 g of commercially available extra-grade rosin, 250 ml of 95% ethanol and 10 ml of concentrated hydrochloric acid into a 500 ml single-necked flask. Heat to reflux for 3 hours, cool to 0°C, let stand for 4 to 5 hours, collect the precipitated solid, wash with distilled water until the washing liquid is neutral, and dry to obtain 75g of abietic acid, wherein the content of abietic acid is close to 95%.
[0041] Add 50g of the abietic acid prepared above into a 500ml three-neck flask, add 60ml of toluene, and stir to dissolve at room temperature. The temperature was controlled at about 40°C, and a mixture of 5ml of concentrated sulfuric acid and 1ml of glacial acetic acid was added dropwise over 1.5 hours, and the reaction was continued for 2.5 hours to complete. Add 200ml of toluene to dilute and let stand for 4 hours to separate the layers. Separate the organic phase and wash it with 0.5% hot (60...
Embodiment 1
[0042] Example 1 Propylenepimaric Acid Reaction with 1,4-Divinyloxymethylcyclohexane (Cyclohexyl Dimethanol Divinyl Ether)
[0043] Add 37.4g (0.1mol) of propylene pimaric acid and 19.6g (0.1mol) of 1,4-divinyloxymethyl ring from reference example 1 in the 500ml four-neck flask equipped with mechanical stirring, thermometer and condenser Add 60ml of xylene to hexane (cyclohexyl dimethyl divinyl ether), heat and stir to dissolve it, raise the temperature to 120-130°C, and stir for 3 hours to complete the reaction.
[0044] Product Determination Infrared Spectrum Visible 3400cm -1 Absorption of nearby carboxylic acids basically disappears, 1690cm -1 Carbonyl absorption of nearby carboxylic acid disappears, 1720cm -1 The carbonyl absorption of the ester group occurs nearby. Indicates that the reaction is complete.
[0045] Example 2 Reaction of Propylene Pimaric Acid with Divinyloxyethyl Ether (Diethylene Glycol Divinyl Ether)
[0046] Add 37.4g (0.1mol) of propylene p...
Embodiment 3
[0048] Example 3 Reaction of Dimeric Abietic Acid with 1,4-Divinyloxymethylcyclohexane (Cyclohexyl Dimethanol Divinyl Ether)
[0049] Add 59.1g (0.1mol) of abietic acid dimer and 19.6g (0.1mol) of 1,4-divinyloxymethyl from reference example 2 in the 500ml four-necked bottle equipped with mechanical stirring, thermometer, and condenser Base cyclohexane (cyclohexyl dimethyl divinyl ether), add 80ml of xylene, heat and stir to dissolve it, heat up to 120-130°C, and stir for 3 hours to complete the reaction.
[0050] Product Determination Infrared Spectrum Visible 3400cm -1 Absorption of nearby carboxylic acids basically disappears, 1690cm -1 Carbonyl absorption of nearby carboxylic acid disappears, 1720cm -1 The carbonyl absorption of the ester group occurs nearby. Indicates that the reaction is complete.
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com