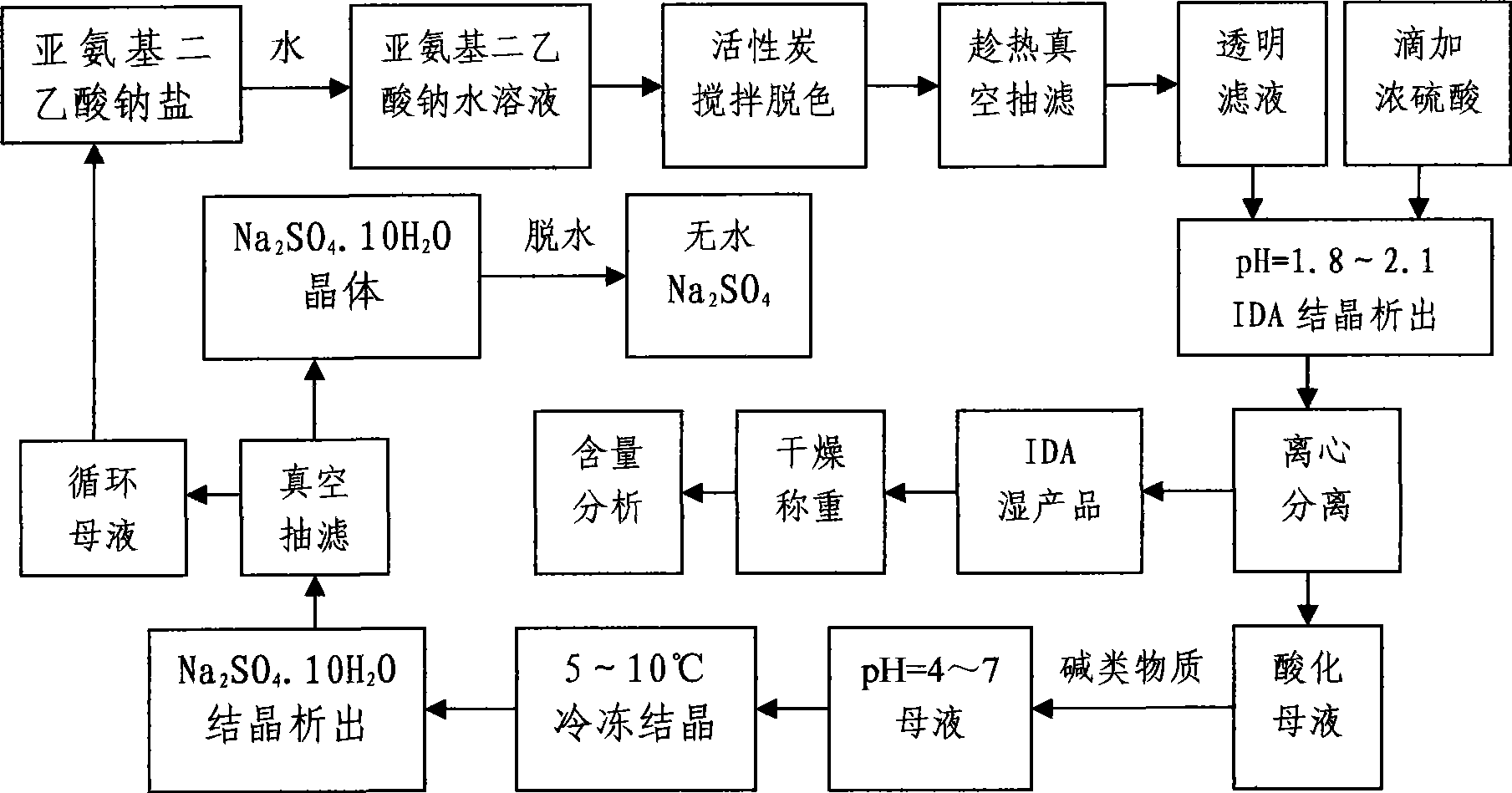
Cycling method for preparing iminodiacetic acid from iminodiacetic acid disodium salt
A technology of sodium iminodiacetic acid and iminodiacetic acid, which is applied in the field of preparation of iminodiacetic acid, can solve the problems of high equipment requirements, high cost, corrosion of ionic membrane, etc., achieve simple and clear process route, improve continuous production capacity, Effects with low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0023] Weigh 500g of sodium iminodiacetate solid, add 1000g of tap water, stir and dissolve in a constant temperature water bath at 70-80°C, then add 5g of activated carbon for decolorization for 20min, vacuum filter while hot to remove impurities to obtain a transparent filtrate, and stir at a constant speed Slowly add about 200g of concentrated sulfuric acid dropwise to adjust pH=1.95, iminodiacetic acid crystallizes out, centrifuge and dry at 100-105°C to obtain IDA product, and acidify the mother liquor to pH=2.5. Add 55g of sodium hydroxide to the acidified mother liquor, adjust the pH=5.25, put it into the freezer for crystallization at 7°C for 5 hours, and precipitate rod-shaped Na 2 SO 4 .10H 2 O, vacuum filtration, and the filtrate was circulated to prepare iminodiacetic acid 30 times. In the experiment, the total yield of IDA was 97.7%, the content was 98.1%, and the salt removal rate was 98.4%.
example 2
[0025] Weigh 500g of sodium iminodiacetate solid, add 1000g of tap water, stir and dissolve in a constant temperature water bath at 70-80°C, then add 5g of activated carbon for decolorization for 20min, vacuum filter while hot to remove impurities to obtain a transparent filtrate, and stir at a constant speed Slowly add about 200g of concentrated sulfuric acid dropwise to adjust pH=1.95, iminodiacetic acid crystallizes out, centrifuge and dry at 100-105°C to obtain IDA product, and acidify the mother liquor to pH=2.5. Add 55g of sodium hydroxide to the acidified mother liquor, adjust the pH=5.25, put it into the freezer for crystallization at 7°C for 5 hours, and precipitate rod-shaped Na 2 SO 4 .10H 2 0, vacuum filtration, and the filtrate was circulated to continuously prepare iminodiacetic acid 40 times. In the experiment, the total yield of IDA was 97.4%, the content was 98.8%, and the salt removal rate was 98.8%.
example 3
[0027] Weigh 500g of sodium iminodiacetate solid, add 1500g of tap water, stir and dissolve in a constant temperature water bath at 70-80°C, then add 5g of activated carbon for decolorization for 20min, vacuum filter while hot to remove impurities to obtain a transparent filtrate, and stir at a constant speed Slowly add about 210g of concentrated sulfuric acid dropwise to adjust pH=1.94, iminodiacetic acid crystallizes out, centrifuges and dries at 100-105°C to obtain IDA product, and acidifies the mother liquor to pH=2.4. Add 60g of sodium hydroxide to the acidified mother liquor, adjust the pH=5.72, put it into the freezer for crystallization at 8°C for 5 hours, and precipitate rod-shaped Na 2 SO 4 .10H 2 O, vacuum filtration, and the filtrate was circulated to continuously prepare iminodiacetic acid 30 times. In the experiment, the total yield of IDA was 97.2%, the content was 98.9%, and the salt removal rate was 98.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com