Cisplatin polymer micelle preparation method and use thereof
A polymer glue and polymer technology, applied in the field of biomedical technology and nanomedicine, can solve the problems of strong toxic and side effects, low oral activity, poor water solubility, etc., and achieve low toxicity, good chemotherapeutic effect, and good passive targeting. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
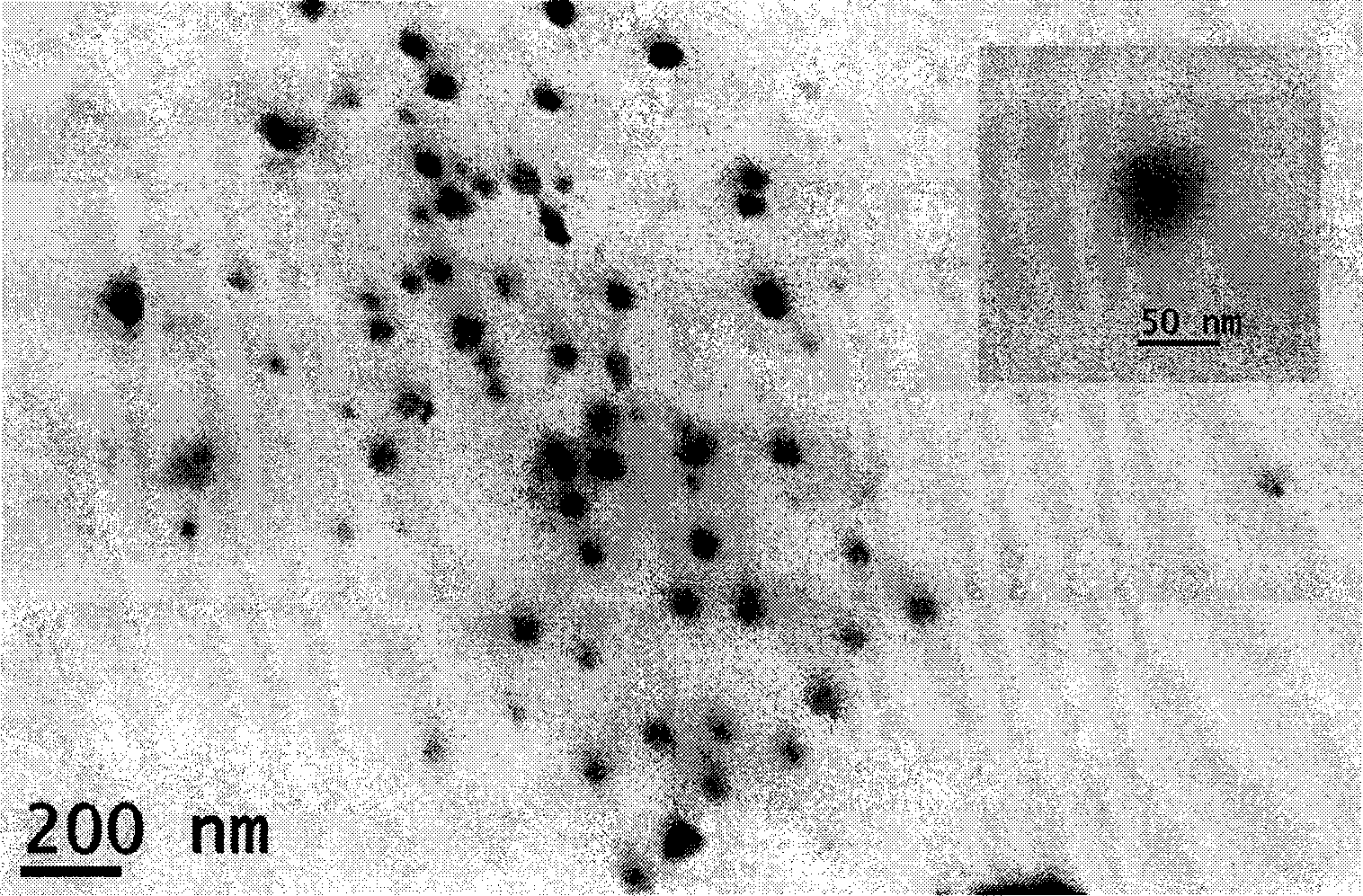
Image
Examples
Embodiment 1
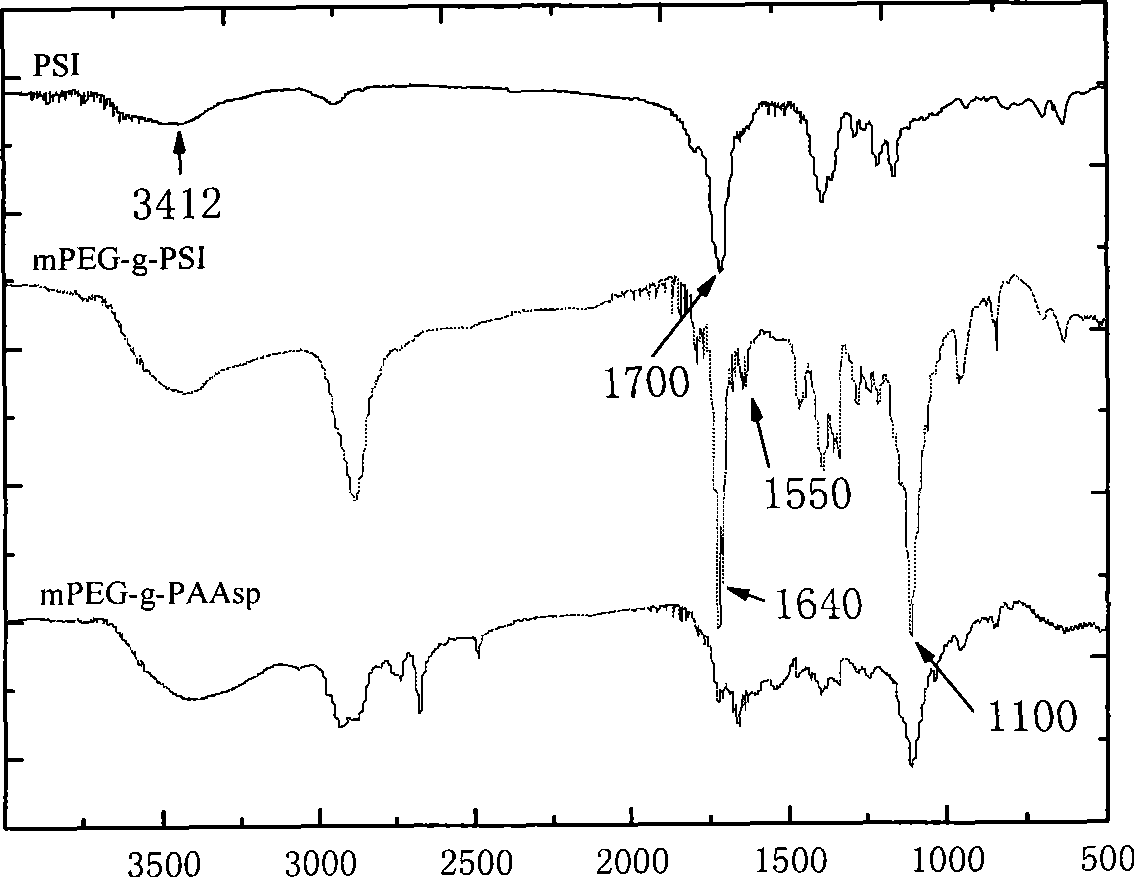
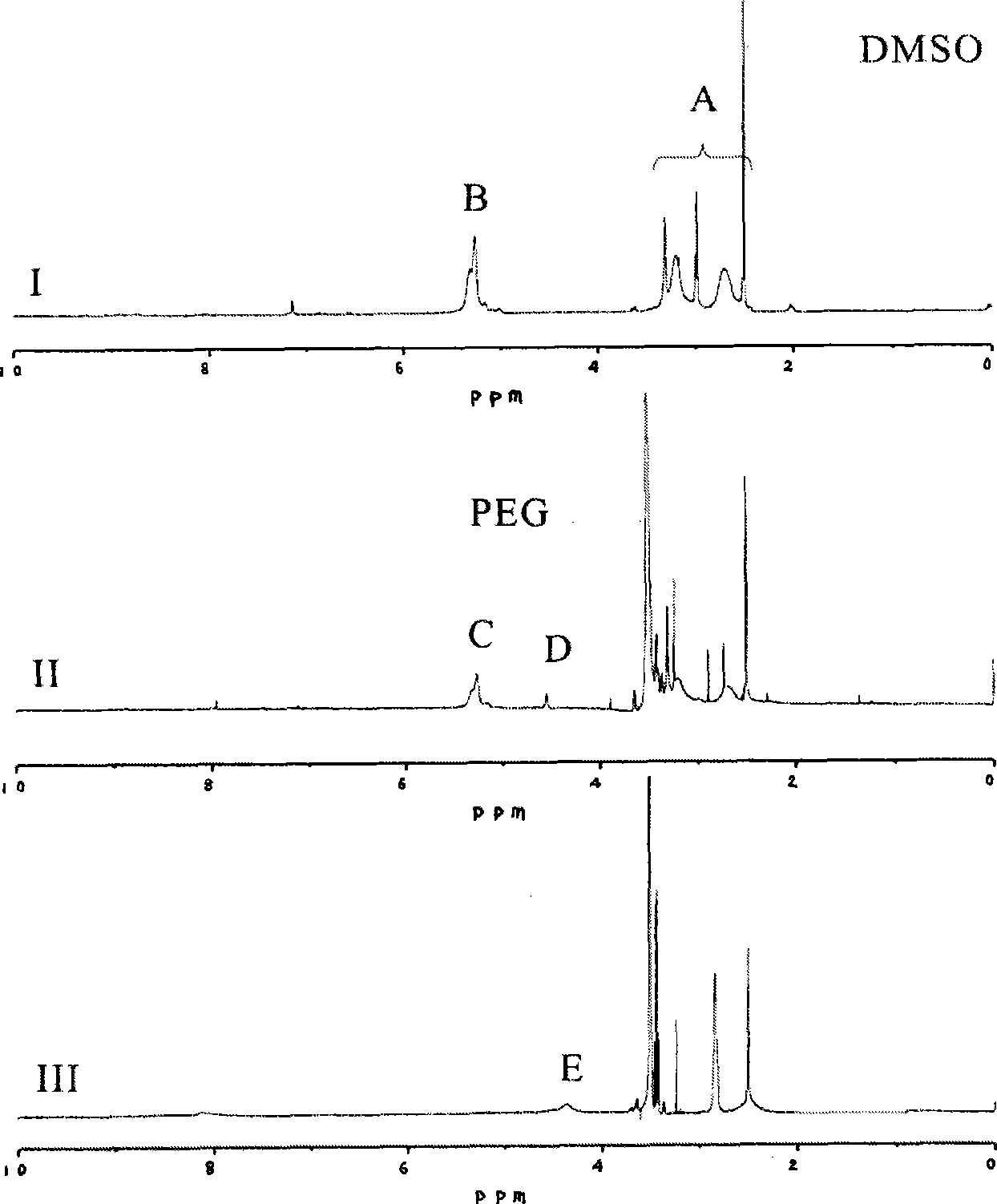
[0028] (a) Synthesis of polyethylene glycol-g-polyaspartimide (mPEG-g-PSI)
[0029] 10g mPEG 5000 -NH 2 Dissolve in 20mL DMF, add dropwise 20mL DMF solution containing 1g polyaspartimide, in N 2 Under protection, heat in an oil bath at 70°C and react for 24h. After reprecipitation with ether and drying in a vacuum, the product polyethylene glycol-g-polyaspartimide can be obtained with a yield of 94%.
[0030] (b) Synthesis of polyethylene glycol-g-polyaspartic acid derivatives (mPEG-g-α, β-Poly[(N-amino acidyl)-DL-aspartamide])
[0031] 2 g of aspartic acid and 1 g of polyethylene glycol-g-polyaspartimide were dissolved in a mixed solvent of 100 mL of water and 20 mL of triethylamine, and reacted at room temperature for 24 hours. The reaction mixture solution was concentrated to 10 mL, adjusted to pH 2 with hydrochloric acid, stirred for 4 hours, dialyzed for 3 days, and freeze-dried to obtain polyethylene glycol-g-polyaspartic acid derivative with a yield of 76%.
[0032] (c) Pre...
Embodiment 2
[0036] (a) Synthesis of polyethylene glycol-g-polyaspartimide (mPEG-g-PSI)
[0037] 4g mPEG 2000 -NH 2 Dissolve in 10mL DMF, add dropwise 20mL DMF solution containing 1g polyaspartimide, in N 2 Under protection, heat in an oil bath at 70°C and react for 24h. The product polyethylene glycol-g-polyaspartimide can be obtained by reprecipitation with ether and drying in a vacuum with a yield of 90%.
[0038] (b) Synthesis of polyethylene glycol-g-polyaspartic acid derivatives (mPEG-g-α, β-Poly[(N-amino acidyl)-DL-aspartamide])
[0039] 4g glutamic acid and 1g polyethylene glycol-g-polyaspartimide were dissolved in a mixed solvent of 80mL water and 10mL triethylamine, and reacted at room temperature for 24h. The reaction mixture solution was concentrated to 10 mL, adjusted to pH 2 with hydrochloric acid, stirred for 4 hours, dialyzed for 3 days, and freeze-dried to obtain polyethylene glycol-g-polyaspartic acid derivative with a yield of 70%.
[0040] (c) Preparation of cisplatin polym...
Embodiment 3
[0044] (a) Synthesis of polyethylene glycol-g-polyaspartimide (mPEG-g-PSI)
[0045] 5g mPEG 2000 -NH 2 Dissolve in 20mL DMF, add dropwise to 10mL DMF solution containing 1g polyaspartimide, in N 2 Under protection, heat in an oil bath at 70°C and react for 24h. The product polyethylene glycol-g-polyaspartimide can be obtained by reprecipitating with ether and drying in a vacuum with a yield of 93%.
[0046] (b) Synthesis of polyethylene glycol-g-polyaspartic acid derivatives (mPEG-g-α, β-Poly[(N-amino acidyl)-DL-aspartamide])
[0047] Dissolve 5 g of aspartic acid and 1 g of polyethylene glycol-g-polyaspartimide in a mixed solvent of 80 mL of water and 10 mL of triethylamine, and react for 24 hours at room temperature. The reaction mixture solution was concentrated to 10 mL, adjusted to pH 2 with hydrochloric acid, stirred for 4 hours, dialyzed for 3 days, and freeze-dried to obtain polyethylene glycol-g-polyaspartic acid derivative with a yield of 73%.
[0048] (c) Preparation of c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com