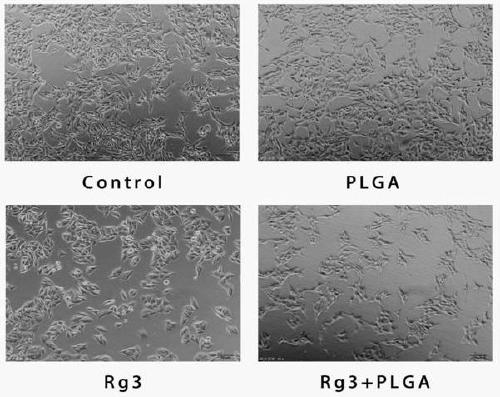
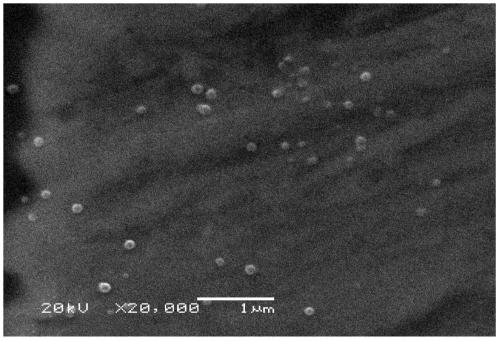
A 20(R)-ginsenoside Rg3 sustained-release nanosphere combination and its preparation method
A technology of ginsenosides and nano-microspheres, which can be used in drug combinations, microcapsules, and pharmaceutical formulations. It can solve the problems of unstudied slow-release nanoparticles, achieve enhanced tissue and plasma protein affinity, and high encapsulation efficiency. , good physical stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] 1. Preparation of polyvinyl alcohol aqueous solution
[0073] Add 1.25g of PVA (polyvinyl alcohol) to 50ml of three-distilled water (water collected after three distillations), heat at 95°C for 2 hours to fully dissolve, filter, and prepare polyvinyl alcohol with a mass-volume concentration of 2.5%. Aqueous solution (50ml), stored at room temperature, for later use;
[0074] 2. Preparation of polylactic acid-glycolic acid copolymer solution (i.e. PLGA oil phase)
[0075] The accurately weighed poly(lactic-co-glycolic acid) copolymer (i.e. PLGA, 0.09g) was dissolved in 2ml of chloroform, stirred evenly, and made into a poly(lactic-co-glycolic acid) solution (i.e. PLGA) with a concentration of 45mg / ml. Oil phase, 2ml) for subsequent use, wherein, the mass percent of lactic acid and glycolic acid in polylactic acid-glycolic acid copolymer is 50:50; Its molecular weight is 6 * 10 3 ;
[0076] 3. Preparation of Rg3-PLGA mixture
[0077] 20(R)-ginsenoside Rg3 (content is ...
Embodiment 2
[0105] 1. Preparation of polyvinyl alcohol aqueous solution
[0106] Add 2.5g of PVA (polyvinyl alcohol) to 50ml of triple-distilled water, heat at 95°C for 2 hours to fully dissolve, filter, and prepare a polyvinyl alcohol aqueous solution (50ml) with a mass volume concentration of 5%, store at room temperature, and set aside;
[0107] 2. Preparation of polylactic acid-glycolic acid copolymer solution (i.e. PLGA oil phase)
[0108] Accurately weighed poly(lactic-co-glycolic acid) (i.e. PLGA, 0.1g) was dissolved in 2ml of chloroform, stirred evenly to make a concentration of 50mg / ml poly(lactic-co-glycolic acid) solution (i.e. PLGA Oil phase, 2ml) for subsequent use, wherein the percentage of lactic acid and glycolic acid in the polylactic acid-glycolic acid copolymer is 60:40, and the molecular weight of the copolymer is 7×10 3 ;
[0109] 3. Preparation of Rg3-PLGA mixture
[0110] 20 (R)-ginsenoside Rg3 (content is 64%, 0.025g) is added to the PLGA oily phase of 2ml, stir...
Embodiment 3
[0126] 1. Preparation of polyvinyl alcohol aqueous solution
[0127] Add 1.5g of PVA (polyvinyl alcohol) to 50ml of triple-distilled water, heat at 95°C for 2 hours to fully dissolve, filter, and prepare a polyvinyl alcohol aqueous solution (50ml) with a mass volume concentration of 3%, store at room temperature, and set aside;
[0128] 2. Preparation of polylactic acid-glycolic acid copolymer solution (i.e. PLGA oil phase)
[0129] Accurately weighed poly(lactic-co-glycolic acid) (i.e. PLGA, 0.12g) was dissolved in 2.5ml of chloroform, stirred evenly, and made into a solution of poly(lactic-co-glycolic acid) with a concentration of 48mg / ml (i.e. PLGA Oil phase, 2.5ml) for subsequent use, wherein the percentage of lactic acid and glycolic acid in the polylactic acid-glycolic acid copolymer is 80:20, and its molecular weight is 8×10 3 ;
[0130] 3. Preparation of Rg3-PLGA mixture
[0131] 20(R)-ginsenoside Rg3 (content is 79%, 0.02g) is added to the PLGA oil phase of 2.5ml, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com