Targeted photothermal therapy combined with immunotherapy anti-tumor compound preparation and its preparation method and application
A technology of photothermal therapy and immunotherapy, applied in the direction of antineoplastic drugs, carrier-bound antigen/hapten components, drug combinations, etc. Time waiting and other issues, to achieve good passive targeting of tumors, good chemical stability, and improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
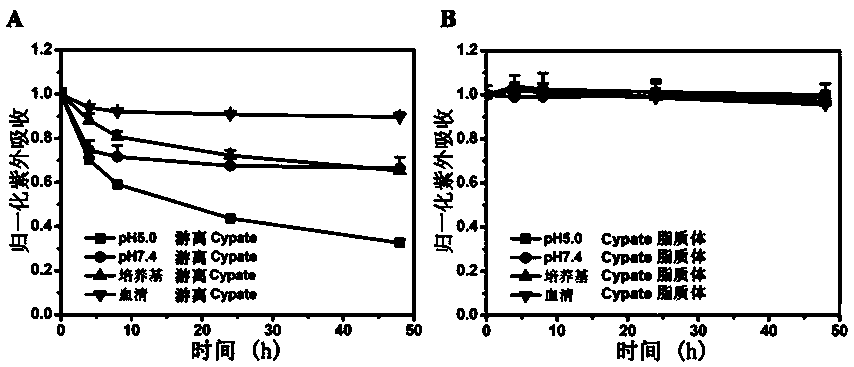
[0054] The investigation of the preparation and physicochemical property of embodiment one Cypate liposome
[0055] Weigh 10g of phospholipids, 2.5g of cholesterol and 0.5g of Cypate into a vial, add 3.75mL of absolute ethanol and 1.25mL of ethyl acetate, seal it, and put it in a water bath at 50°C for 1250 r min -1 Stir for 5 min and sonicate for 1 min to fully dissolve it. Quickly and evenly add 430g of purified water under stirring condition, 50℃ water bath 1250 r min -1 Stir for 5 minutes to emulsify, sonicate for 1 minute, and continue stirring for 15 minutes to make the emulsification complete. Open the lid and put it in a 37°C water bath at 750 r min -1 Stir for 5 hours, completely volatilize the organic solvent, and obtain the Cypate liposome suspension.
[0056] The prepared Cypate liposomes have good uniformity, and the shape is regular spherical, and the apparent morphology of Cypate liposomes observed by a transmission electron microscope (TEM) is spherical and e...
Embodiment 2
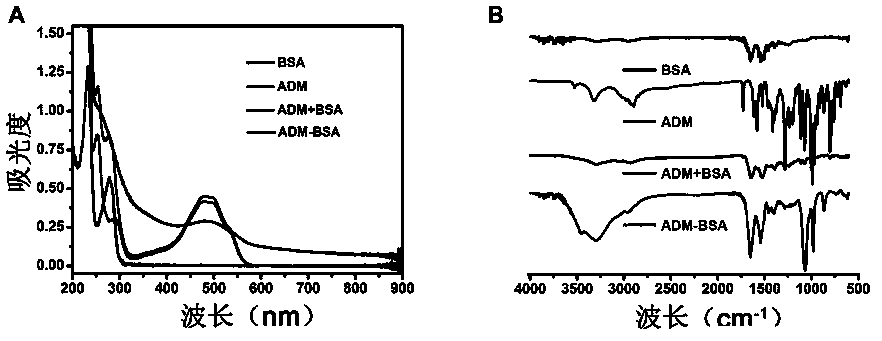
[0066] Example 2 Synthesis of Immunogen BSA-ADM and Preparation of Antibody
[0067] Doxorubicin is a small molecule with only immunoreactivity but no immunogenicity. It cannot directly immunize animals to produce antibodies. It needs to be coupled with heterologous proteins (mostly bovine serum albumin, BSA) to prepare immunogens. In addition, in the enzyme-linked immunosorbent assay (ELISA) method, the antibody is bound to the coated antigen on the microtiter plate, and doxorubicin is often combined with another variant protein (multipurpose ovalbumin, OVA).
[0068] Synthetic immunogen ADM-BSA and coating agent ADM-OVA were prepared by glutaraldehyde cross-linking method. The preparation process is as follows: Weigh 100 mg of BSA into a 25 mL round bottom flask, add 5 mL of 0.1 mol . L -1 PBS solution, fully dissolved, stirring at 10 r . min -1 , another 40.0 mg ADM . HCl was dissolved in 3.0 mL DMF and PBS mixed solution (DME:PBS=1:1), ADM was added dropwise to the BS...
Embodiment 3
[0086] The synthesis of embodiment three HA-ADM
[0087] The synthetic route is as follows:
[0088] (1) Activation of hyaluronic acid: Weigh 42.07 mg of hyaluronic acid (HA) into a 10 mL round bottom flask, add 2.0 mL of purified water, fully dissolve, and stir at 120 r min -1 , another 38.3 mg EDC and 22.9 mg NHS were added sequentially, and reacted for 8 hours under dark conditions, 8000 r min -1 After centrifugation for 10 min, the supernatant was collected, which was the activated HA solution.
[0089] (2) Synthesis of HA-ADM: take 10.8 mg ADM . After HCl was dissolved in 1.3 mL of purified water, it was added dropwise to the above activated HA solution, 120 r min -1 , and react in the dark at 25°C for 12 h. 8000 rpm -1 Centrifuge for 10 min to take the supernatant, put it in a dialysis bag with a molecular cut-off of 8000-14000, use 1000 mL purified water as the receiving solution, dialyze, change the receiving solution every 24 hours, after the dialysis is complete...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com