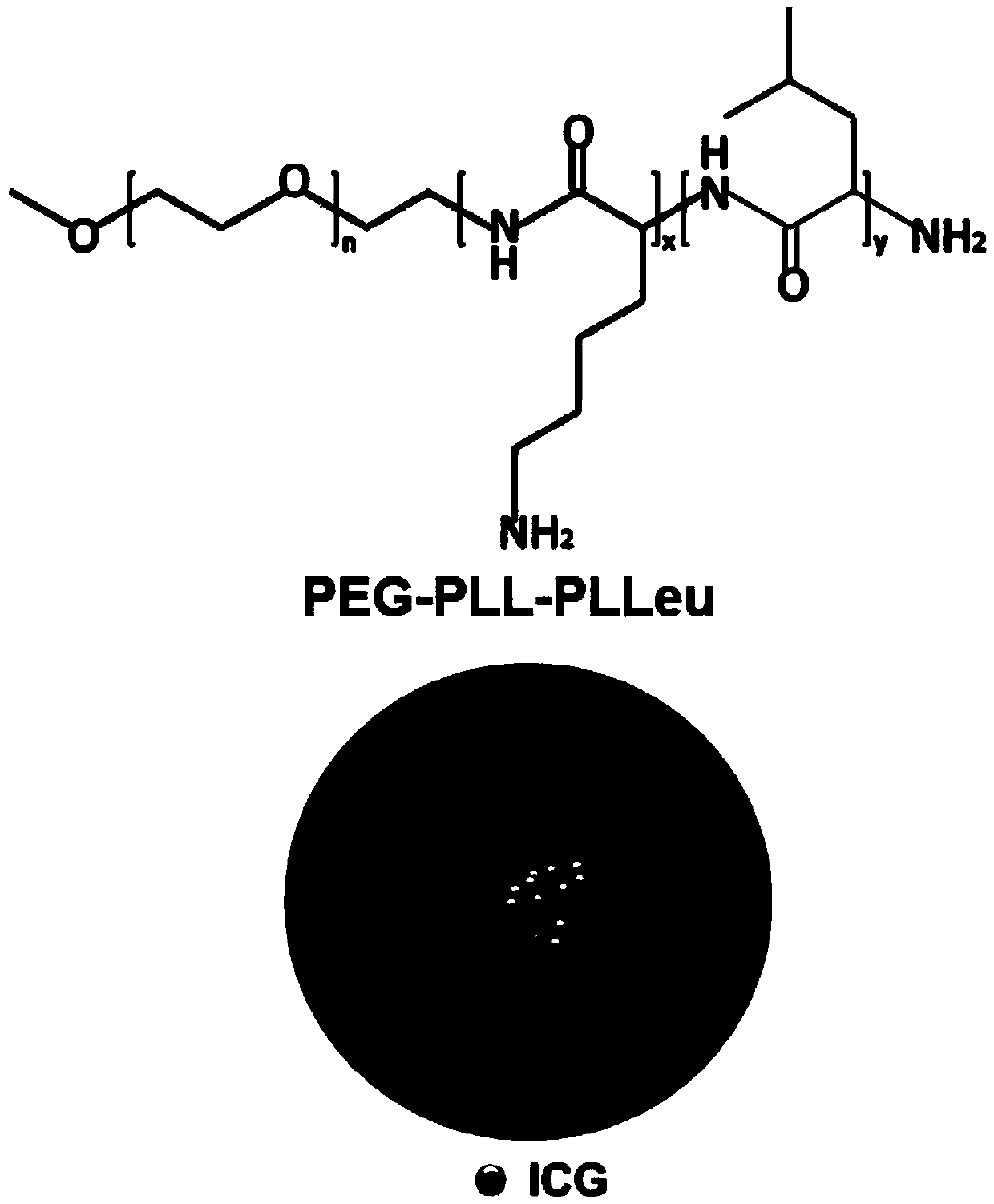
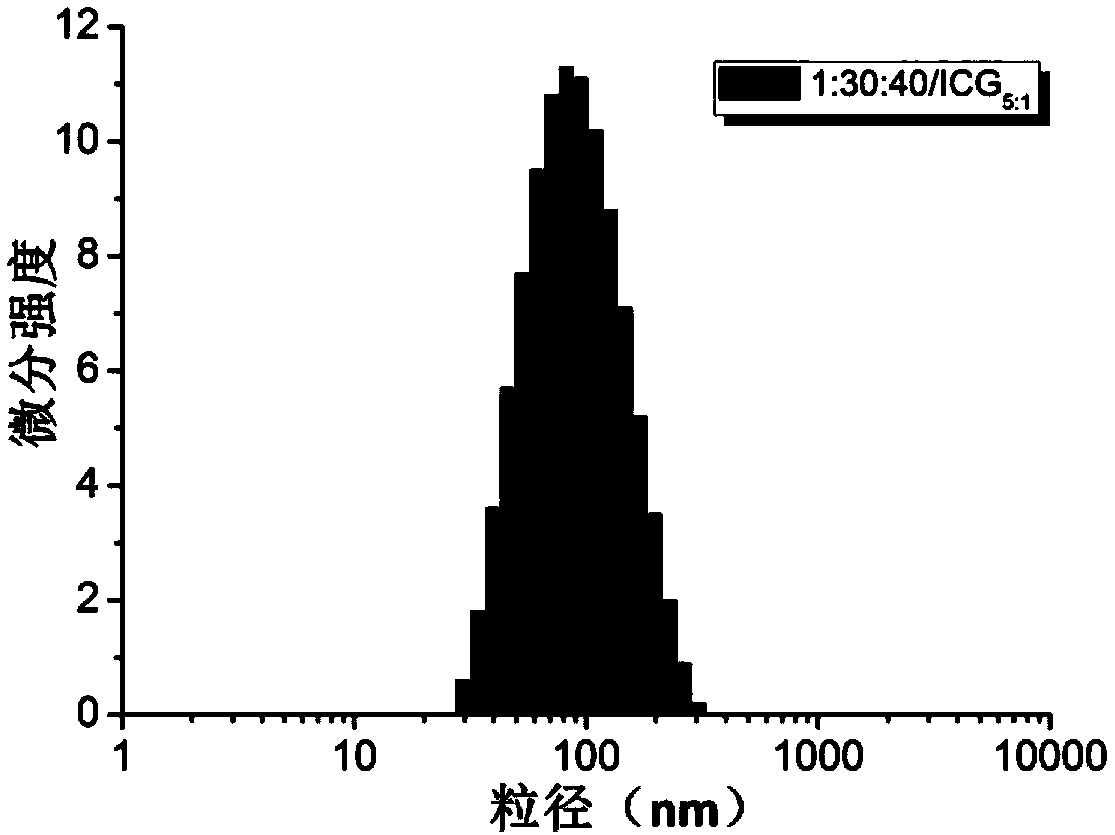
Amphipathy tri-block polypeptide ICG (Indocyanine Green) loaded micelle and preparation method thereof
A technology of polypeptide and triblock, which is applied in the field of nanomaterials to achieve the effects of enhanced half-life, less damage to organisms, and enhanced stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] (1) Synthesis of amino acid anhydride (NCA), including the synthesis of lysine anhydride Lys(Z)-NCA and leucine anhydride Leu-NCA:
[0048] Synthesis of Lys(Z)-NCA: Add Lys(Z) (10g, 33.56mmol) and 100mL tetrahydrofuran THF to a dry reaction flask, stir, and heat the oil bath to 50°C, and triphosgene (4.6g, 97.71mmol) is completely dissolved After adding 20mL of THF into the constant pressure dropping funnel, after the temperature of the reaction solution was stabilized, dropwise (1 drop / second) the triphosgene solution, the reactant immediately became gelatinous, and could not be stirred at this time. After 30 minutes, the gel was slowly dissolved with the dripping of the triphosgene solution, and at this time, it began to stir slowly at a frequency of 100 rpm. During the reaction, the N 2 , reacted for 3 hours, and continued to react for 30 minutes after the reaction solution completely turned into a clear and slightly yellow solution, so as to allow the reaction to c...
Embodiment 2
[0054] (1) Synthesis of amino acid anhydride (NCA), including the synthesis of lysine anhydride Lys(Z)-NCA and leucine anhydride Leu-NCA: the same as (1) in Example 1.
[0055] (2) MPEG-b-PZLL 20 Synthesis: By combining amino-containing PEG (MPEG-NH 2 ) and lysine anhydride Lys(Z)-NCA react with a molar ratio of 1:20 to obtain MPEG-b-PZLL 20 : First MPEG-NH 2 (0.33g, 0.16mmol) was dissolved in 10mL of dry DMF as a macroinitiator to obtain reaction solution A; Lys(Z)-NCA (1g, 3.2mmol) from step (1) was dissolved in 10mL of DMF , to obtain the reaction solution B; then the reaction solution A and the reaction solution B are mixed, in N 2 , heated in an oil bath and stirred at a rate of 300rpm, reacted at 30°C for 72 hours, and the reaction solution became a colorless transparent viscous liquid; after the reaction was completed, the product was dropped dropwise into 100mL of anhydrous ether stirred at 400rpm, and a white precipitate was obtained. Centrifuge at 25°C and 5000rp...
Embodiment 3
[0059] (1) Synthesis of amino acid anhydride (NCA), including the synthesis of lysine anhydride Lys(Z)-NCA and leucine anhydride Leu-NCA: the same as (1) in Example 1.
[0060] (2) MPEG-b-PZLL 30 Synthesis: By combining amino-containing PEG (MPEG-NH 2 ) and lysine anhydride Lys(Z)-NCA react with a molar ratio of 1:30 to obtain MPEG-b-PZLL 30 : First MPEG-NH 2 (0.33g, 0.16mmol) was dissolved in 10mL of dry DMF as a macroinitiator to obtain reaction solution A; Lys(Z)-NCA (1.5g, 4.8mmol) from step (1) was dissolved in 10mL of DMF In, the reaction solution B is obtained; then the reaction solution A and the reaction solution B are mixed, and in N 2 , heated in an oil bath and stirred at a rate of 300rpm, reacted at 32°C for 60h, and the reaction solution became a colorless transparent viscous liquid; after the reaction was completed, the product was dropped dropwise into 100mL of anhydrous ether stirred at 400rpm, and a white precipitate was obtained. Centrifuge at 25°C and 5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com