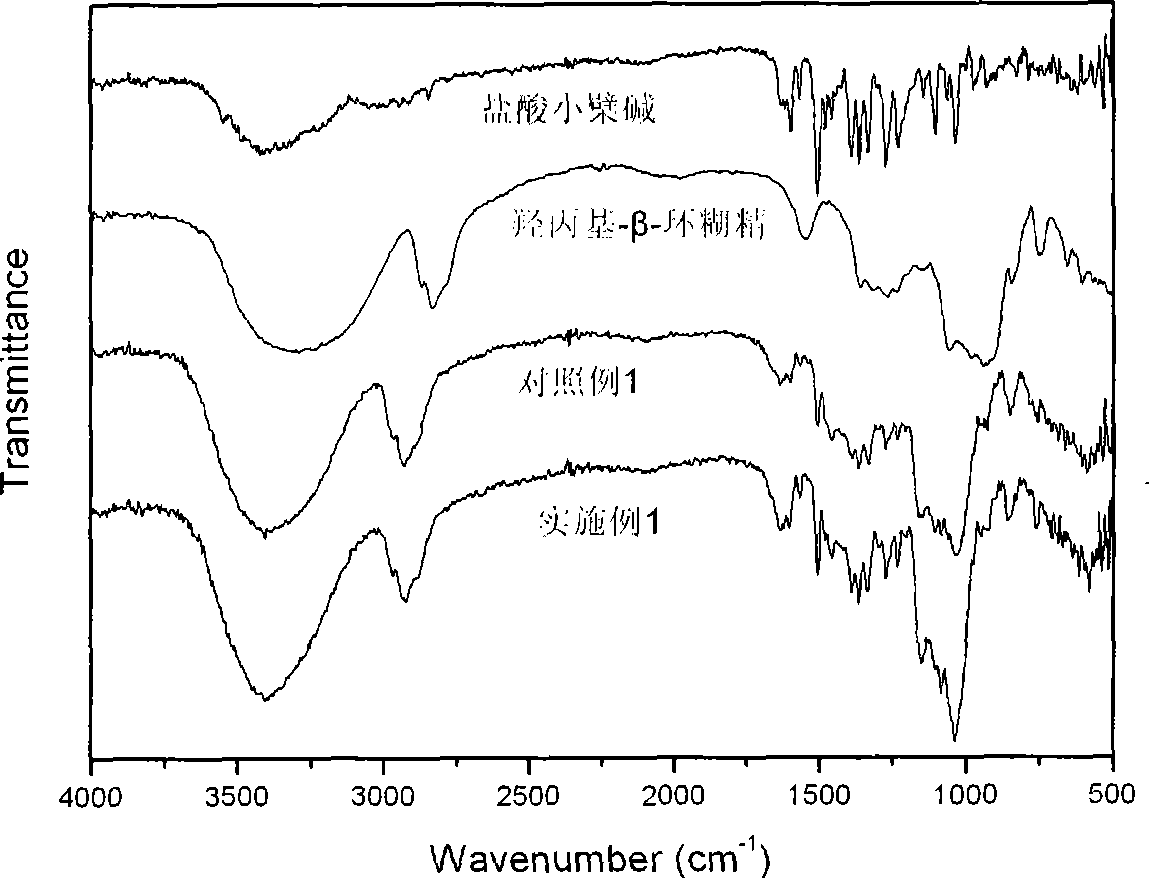
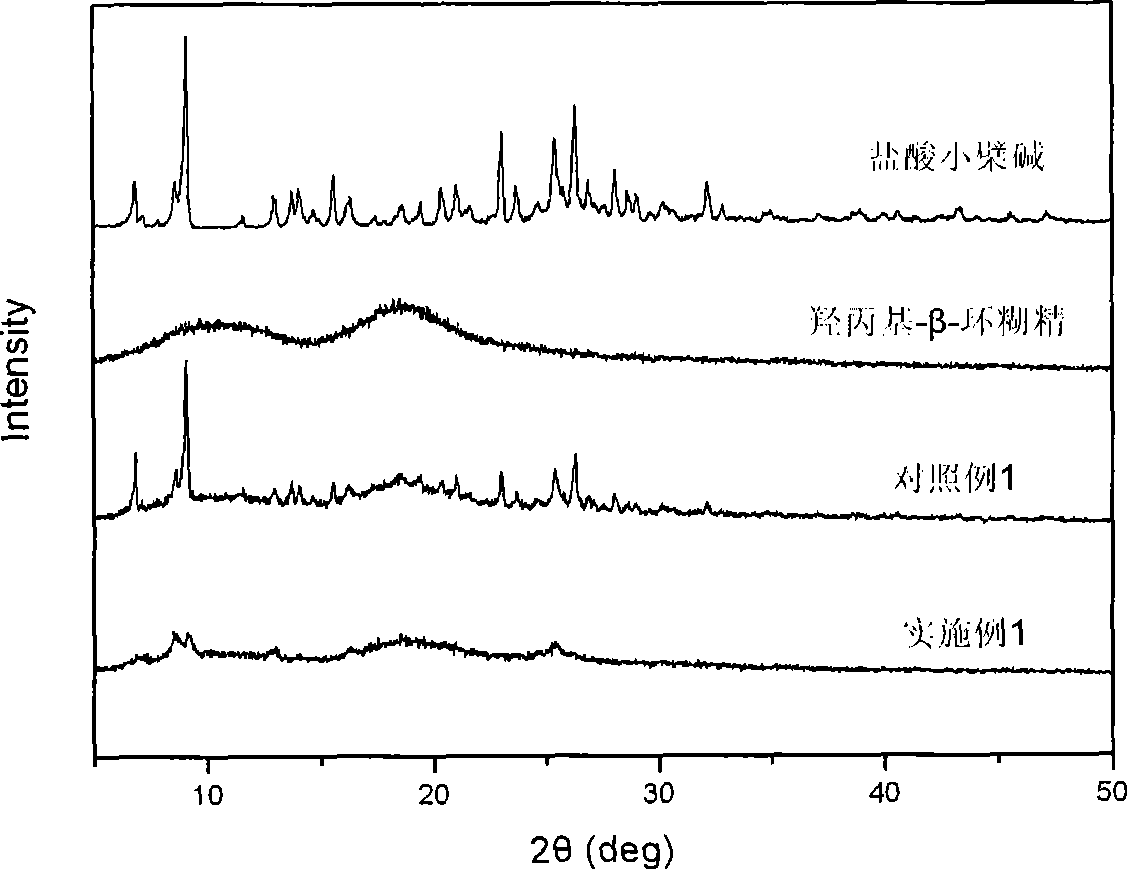
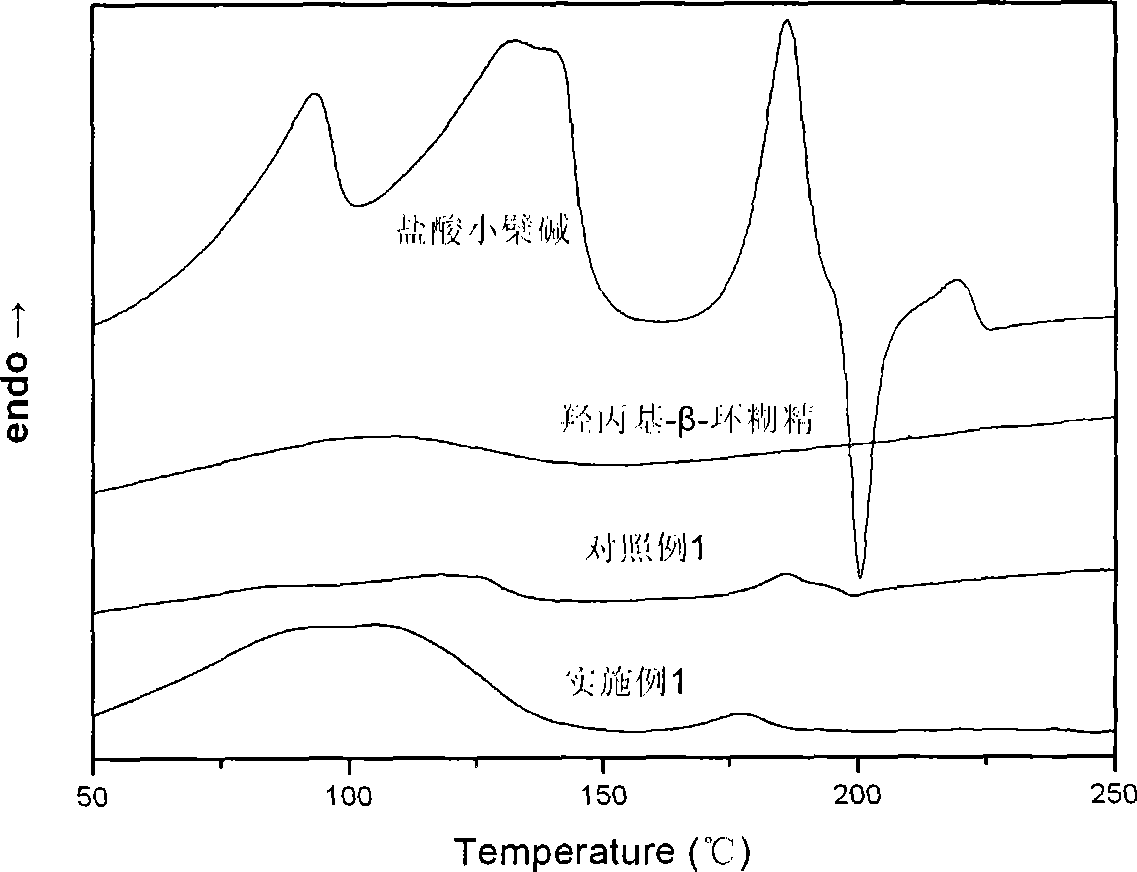
Berberine cyclodextrin inclusion compound, preparation thereof and preparation method
A cyclodextrin inclusion compound and cyclodextrin technology are applied in the field of pharmaceutical preparations of heterocyclic systems and their preparation, and can solve the problems of inclusion compound encapsulation efficiency, low drug loading and purity, encapsulation efficiency, loading and unloading. Low dosage and purity, low bioavailability of inclusion complexes, etc., to achieve the effect of large effective permeability coefficient, avoid secondary infection, and promote inclusion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 1. Preparation of berberine hydrochloride / hydroxypropyl-β-cyclodextrin inclusion compound
[0044] 1) Put 2g of hydroxypropyl-β-cyclodextrin in a beaker, add 1L of deionized water, and stir to dissolve;
[0045] 2) After heating the hydroxypropyl-β-cyclodextrin solution to 60°C, slowly add 574 mg of berberine hydrochloride solution dissolved in a small amount of absolute ethanol to the hydroxypropyl-β-cyclodextrin while stirring In the solution, the stirring rate is 800rpm. After stirring for 12 hours, the temperature is gradually lowered at a rate of 5°C / h, and the temperature is slowly lowered to 25°C;
[0046] 3) Filter the cooled solution with a 0.22 μm pore size microporous filter (to remove unincluded berberine hydrochloride);
[0047] The filtrate is placed in a spray dryer for spray drying. The inlet temperature of the filtrate spray dryer is 145° C., and the temperature of the sample outlet is 75° C. to obtain a white loose powder, namely berberine hydrochlori...
Embodiment 2
[0057] 1. Preparation of berberine hydrochloride / hydroxypropyl-β-cyclodextrin inclusion compound
[0058] 1) Put 1g of hydroxypropyl-β-cyclodextrin in a beaker, add 1L of deionized water, stir, and heat to 45°C to dissolve it;
[0059] 2) After heating the hydroxypropyl-β-cyclodextrin solution to 45°C, slowly add 574 mg of berberine hydrochloride solution dissolved in a small amount of absolute ethanol to the hydroxypropyl-β-cyclodextrin while stirring In the solution, the stirring rate is 800rpm. After stirring for 24 hours, the temperature is gradually lowered at a rate of 3°C / h, and the temperature is slowly lowered to 20°C;
[0060] 3) Filter the cooled solution with a 0.22 μm pore size microporous filter (to remove unincluded berberine hydrochloride);
[0061] 4) Put the filtrate in a freeze dryer for freeze-drying under reduced pressure. The freeze-drying temperature is -45°C and the relative pressure is -0.06MPa to obtain a white and loose powder, namely berberine hydr...
Embodiment 3
[0066] 1. Preparation of berberine hydrochloride / hydroxypropyl-β-cyclodextrin inclusion compound
[0067] 1) Put 6g of hydroxypropyl-β-cyclodextrin in a beaker, add 2L of deionized water, and stir to dissolve;
[0068] 2) After heating the hydroxypropyl-β-cyclodextrin solution to 50°C, slowly add 574 mg of berberine hydrochloride solution dissolved in a small amount of absolute ethanol to the hydroxypropyl-β-cyclodextrin while stirring In the solution, the stirring rate is 800rpm. After stirring for 12 hours, the temperature is gradually lowered at a rate of 6°C / h, and the temperature is slowly lowered to 25°C;
[0069] 3) Filter the cooled solution with a 0.22 μm pore size microporous filter (to remove unincluded berberine hydrochloride);
[0070] 4) Put the filtrate in a freeze dryer for freeze-drying under reduced pressure. The freeze-drying temperature is -45°C and the relative pressure is -0.06MPa to obtain a white and loose powder, namely berberine hydrochloride / hydroxy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com