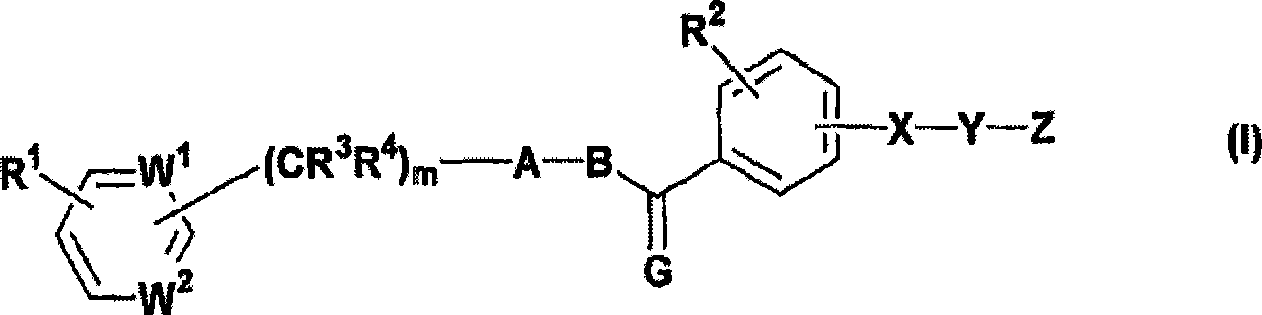
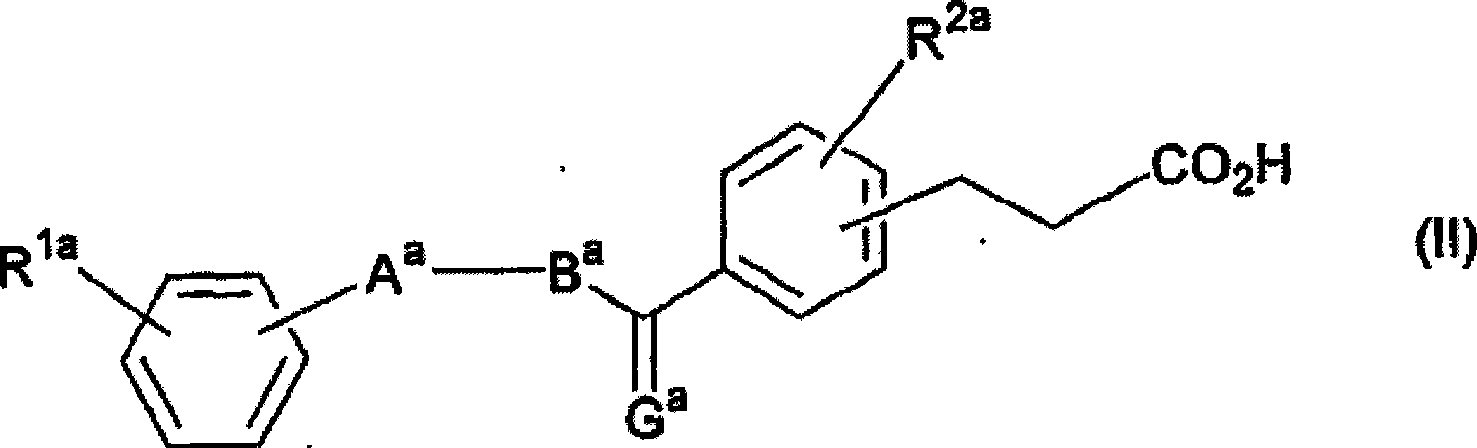
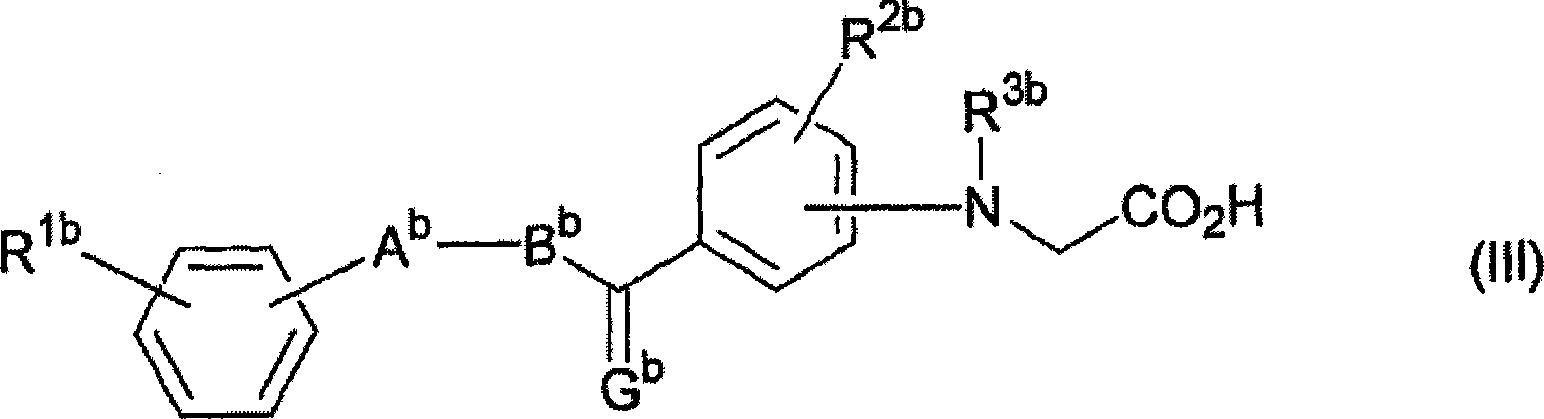
Activating agent for peroxisome proliferator activated receptor delta
A compound and alkoxy technology, applied in the field of activators, can solve problems such as undocumented efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0250] 3-[4-[3-[4-Hexyl-2-(4-methylphenyl)thiazol-5-yl]propanoyl]-2-methylphenyl]propanoic acid
[0251] (1) 4-hexyl-2-(4-methylphenyl)thiazole-5-carbaldehyde
[0252] Dissolve [4-hexyl-2-(4-methylphenyl)thiazol-5-yl]methanol (500 mg, 1.727 mmol) in anhydrous dichloromethane (6 ml), add molecular sieves (3A powder, 890 mg) and Corey reagent (crorochrome) piridium) (745mg, 3.455mmol). After stirring at room temperature for 30 minutes, diethyl ether (20 mL) and silica gel (Wako-gel, C-300HG, 2 g) were added and stirred at room temperature for further 10 minutes. The reaction mixture was filtered through a glass filter, and the residue was washed with diethyl ether to obtain a filtrate, from which the solvent was distilled off under reduced pressure. The resulting residue was subjected to silica gel column chromatography to obtain the title compound (346 mg, yield 70%) as white crystals from the hexane:ethyl acetate (8:1, v / v) fraction.
[0253] 1 H NMR (CDCl 3 , 400MHz)...
Embodiment 2
[0340] 3-[4-[3-[3-isopropyl-5-[4-(trifluoromethyl)phenyl]thiophen-2-yl]propionyl]-2-methylphenyl] propionic acid
[0341] (1) 3-isopropyl-5-[4-(trifluoromethyl)phenyl]thiophene-2-carbaldehyde
[0342] Using [3-isopropyl-5-[4-(trifluoromethyl)phenyl]thiophen-2-yl]methanol, the title compound was obtained in the same manner as in Example 1(1).
[0343] light yellow crystal
[0344] Yield 57%
[0345] 1 H NMR (CDCl 3 , 400MHz): δ=
[0346] 1.39(6H,d,J=7Hz),
[0347] 3.6—3.8(1H,m),
[0348] 7.37(1H,s),
[0349] 7.6.8 (2H, d, J = 8Hz),
[0350] 7.77 (2H, d, J = 8Hz),
[0351] 10.11(1H, s).
[0352] (2) 3-[4-[3-[3-isopropyl-5-[4-(trifluoromethyl)phenyl]thiophen-2-yl]acryloyl]-2-methylphenyl]acrylic acid methyl ester
[0353] The title compound was obtained in the same manner as in Example 1(5).
[0354] yellow crystal
[0355] Yield 67%
[0356] 1 H NMR (CDCl 3 , 400MHz): δ=
[0357] 1.32(6H,d,J=7Hz),
[0358] 2.54(3H,s),
[0359] 3.3—3.5(1H,m),
[0360] 3.8...
Embodiment 3
[0402] 3-[4-[3-(5-Isopropyl-2-phenyl-4-oxazolyl)propionyl]-2-methylphenyl]propanoic acid
[0403] (1) 5-isopropyl-2-(2,4-dichlorophenyl)oxazole-4-carbaldehyde
[0404] Using 5-isopropyl-2-(2,4-dichlorophenyl)oxazole-4-methanol, the title compound was obtained in the same manner as in Example 1(1).
[0405] light yellow crystal
[0406] 1 H NMR (CDCl 3 , 400MHz): δ=
[0407] 1.39(6H,d,J=7Hz),
[0408] 3.72 (1H, dq, J=7, 7Hz),
[0409] 7.37 (1H, dd, J=2, 8Hz),
[0410] 7.5.5 (1H, d, J = 2Hz),
[0411] 7.99 (1H, d, J = 8Hz),
[0412] 10.06(1H, s).
[0413] (2) 3-[4-[3-[5-isopropyl-2-(2,4-dichlorophenyl)-4-oxazolyl]acryloyl]-2-methylphenyl]methacrylate ester
[0414] Use the above-mentioned 5-isopropyl-2-(2,4-dichlorophenyl) oxazole-4-formaldehyde and 3-(4-acetyl-2-methylphenyl) methyl acrylate, adopt and The title compound was obtained in the same manner as in Example 1(5).
[0415] light yellow crystal
[0416] 1 H NMR (CDCl 3 , 400MHz): δ=
[0417] 1.39(6H,d,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com