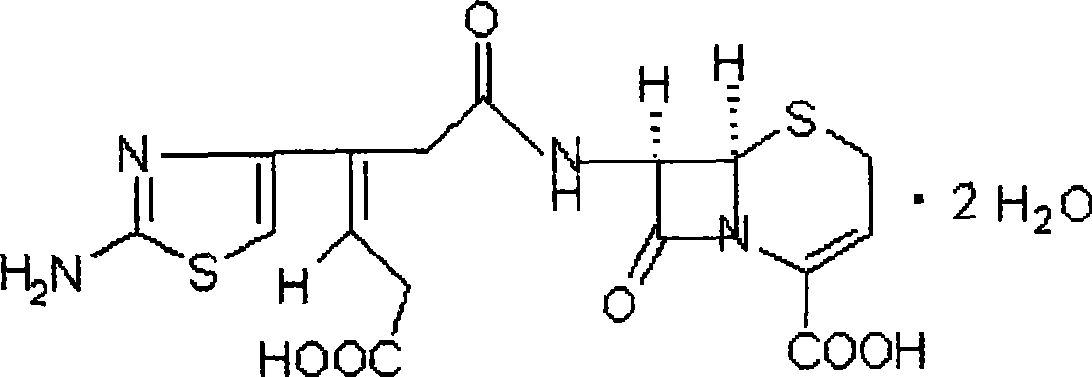
Ceftibuten dispersible tablet
A technology of cefbutene and dispersible tablets, which is applied in the new field of medicine, can solve problems such as inconvenience, and achieve the effects of high quality, simple method and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: specification 1000 pieces
[0031] prescription:
[0032] Ceftibuten 200g
[0033] Microcrystalline Cellulose 280g
[0034] Starch 100g
[0035] Sodium carboxymethyl starch 120g
[0036] Aspartame 3g
[0037] Strawberry essence 2g
[0038] Micronized silica gel 8g
[0040] Preparation method:
[0041] Take by weighing ceftibuten, microcrystalline cellulose, starch, half amount of sodium carboxymethyl starch, cross 80 mesh sieves and mix uniformly, granulate with 18 mesh sieves of 50% ethanol, add the other half amount of sodium carboxymethyl starch, Micronized silica gel, aspartame, strawberry essence, magnesium stearate, passed through a 20-mesh sieve for granulation, mixing, intermediate inspection, tableting.
Embodiment 2
[0042] Embodiment 2: specification 10000 pieces
[0043] prescription:
[0044] Ceftibuten 2000g
[0045] Microcrystalline Cellulose 2800g
[0046] Starch 1000g
[0047] Sodium Carboxymethyl Starch 1200g
[0048] Aspartame 30g
[0049] Strawberry essence 20g
[0050] Micronized silica gel 80g
[0052] Preparation method:
[0053]Take by weighing ceftibuten, microcrystalline cellulose, starch, half amount of sodium carboxymethyl starch, cross 80 mesh sieves and mix uniformly, granulate with 18 mesh sieves of 50% ethanol, add the other half amount of sodium carboxymethyl starch, Micronized silica gel, aspartame, strawberry essence, magnesium stearate, passed through a 20-mesh sieve for granulation, mixing, intermediate inspection, tableting.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com