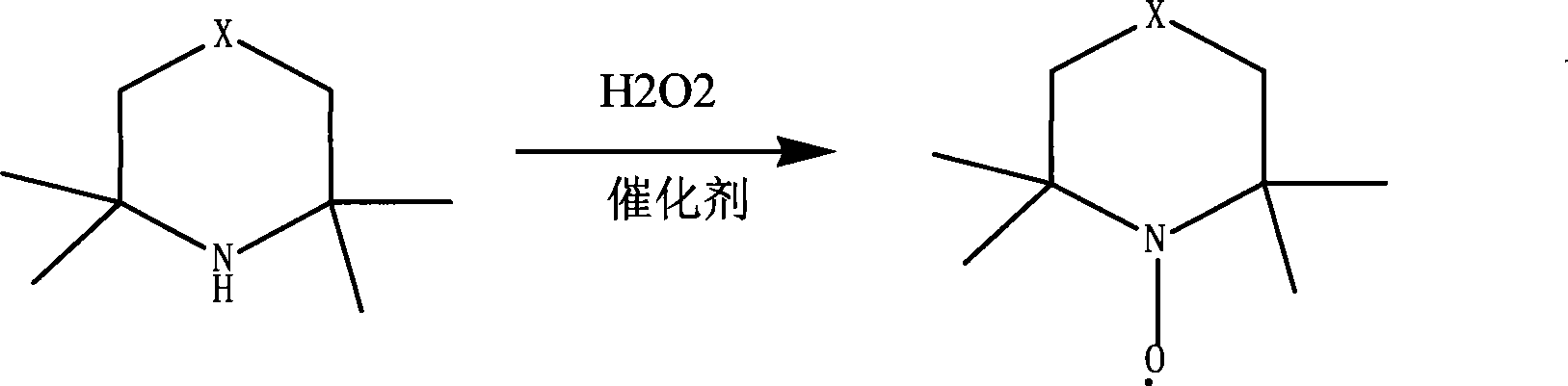
Preparation of piperidine nitroxyl radical anti-polymerization inhibitor
A technology of nitroxide free radicals and piperidines, which is applied in the field of preparation of anti-polymerization inhibitors, can solve the problems of less repetitions, long reaction time, and difficult separation, and achieve mild reaction conditions, short reaction time, and easy separation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1 of the present invention comprises the following steps:
[0025] 1) Add 500g of water, 500g (3.55mol) of 2.2.6.6-tetramethylpiperidine, 50mg of magnesium oxide into a 2L three-necked reaction flask, stir at 75°C, and drop 850g (7.5mol) of the mixture in one hour using the dropping funnel The hydrogen peroxide with a concentration of 30% was reacted for four hours, and the gas phase detection showed that the conversion rate of the reaction liquid reached more than 99.8%, and the reaction was stopped;
[0026] 2) Suction filtration, separate magnesium oxide, reclaim direct utilization;
[0027] 3) After the filtrate was concentrated to dryness under reduced pressure at around 75°C, the scraper was scraped to obtain 552g orange-red finished product, with a molar yield of 99.87%, a gas phase content of 99.52%, a melting point of 37.8-38.9, a residue on ignition of less than 0.03%, and a conversion rate of 100%.
Embodiment 2
[0029] Embodiment 2 of the present invention comprises the following steps:
[0030] 1) Add 500g of water, 500g of 2.2.6.6-tetramethylpiperidine, and 50mg of magnesium oxide in a 2L three-necked reaction flask, stir at 20°C, and use the dropping funnel to drop 605g of 30% hydrogen peroxide in one hour, and react After 3 hours, the conversion rate of the reaction liquid content reached more than 99.8%, and the reaction was stopped;
[0031] 2) Suction filtration, separate magnesium oxide, reclaim direct utilization;
[0032] 3) After the filtrate was concentrated to dryness under reduced pressure at around 20°C, the scraper was scraped to obtain 553g orange-red finished product, with a molar yield of 99.84%, a gas phase content of 99.5%, a melting point of 37.4-38.8, a residue on ignition of less than 0.03%, and a conversion rate of 100%.
Embodiment 3
[0034] Embodiment 3 of the present invention comprises the following steps:
[0035] 1) Add 500g of water and 500g of 2.2.6.6-tetramethylpiperidine, 2.5g of magnesium sulfate in a 5L three-necked reaction flask, stir at 100°C, and use the dropping funnel to drop 1610g of 30% hydrogen peroxide in one hour. After reacting for 15 hours, the conversion rate of the reaction liquid content reached more than 99.8%, and the reaction was stopped;
[0036] 2) Suction filtration, separate magnesium sulfate, reclaim direct utilization;
[0037] 3) After the filtrate was concentrated to dryness under reduced pressure at about 100°C, the scraper was scraped to obtain 554g orange-red finished product, the molar yield was 99.89%, the gas phase content was 99.57%, the melting point was 37.4-38.8, the residue on ignition was less than 0.03%, and the conversion rate was 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com