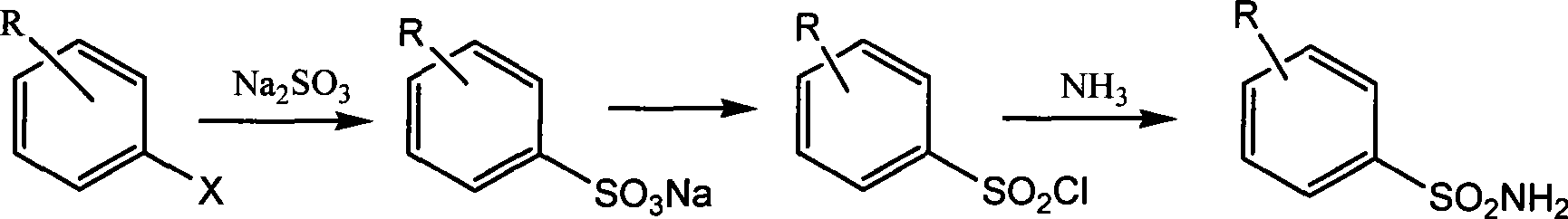
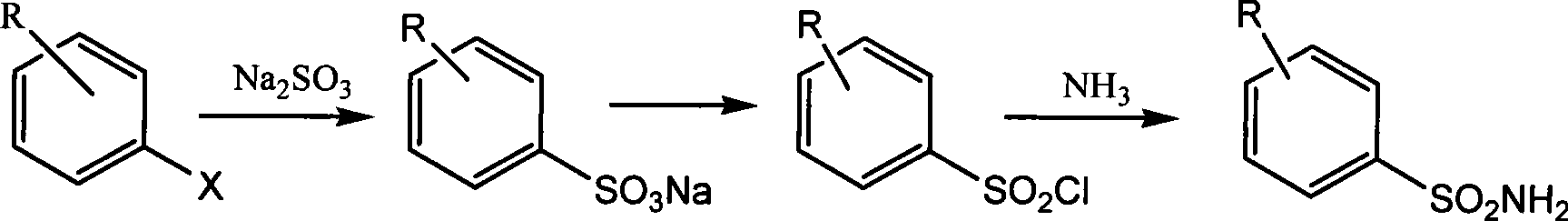
Preparation of alkyl pyridine-2-sulfonic acid amide
A technology of alkylpyridine and sulfonamide, applied in the direction of organic chemistry, etc., can solve problems such as being unsuitable for mass production, complicated reaction process and post-processing process, etc., and achieve the effects of less three wastes, mild reaction conditions, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Embodiment 1: the preparation of 5-picoline-2-sodium sulfonate
[0014] 65g (0.51mol) of sulfurous acid was dissolved in 300mL of water and added to a 500mL reactor, and then 60g of 2-chloro-5-methylpyridine (0.47mol) was added to the reactor. After sealing the kettle, start stirring (rotating speed 160 rev / min), and heat up to 190° C. (pressure reaches 2 MPa). After stirring and reacting for 20 hours, the temperature was lowered to room temperature, and the reaction mixture was taken out, concentrated, and dried to obtain an off-white solid (containing inorganic salt).
Embodiment 2
[0015] Embodiment 2: Preparation of 5-picoline-2-sulfonyl chloride
[0016] Add 112 g of the crude product 5-picoline-2-sulfonate sodium obtained in the previous step, 200 ml of thionyl chloride and 2 ml of N,N-dimethylimine into a 500 mL reaction flask. The reaction mixture was heated to reflux, stirred for 4 hours, and cooled to room temperature. The reaction solution was concentrated to dryness to obtain a reddish-brown oil.
Embodiment 3
[0017] Embodiment 3: the preparation of 5-picoline-2-sulfonamide
[0018] The oil obtained in the previous step was transferred to a 500 mL three-neck reaction flask with 200 mL of dichloromethane, and the reaction flask was cooled in ice water. Slowly add 150 ml of concentrated ammonia water dropwise under stirring, keeping the reaction temperature below 5°C. After the addition was complete (about 0.5 hours), warm to room temperature and continue stirring for 3.0 hours. The organic layer was separated, and the aqueous layer was extracted three times with 300 mL of dichloromethane. The organic layer and the extract were combined, washed with 100 mL of saturated brine, dried over anhydrous sodium sulfate, concentrated to obtain a yellow solid, and recrystallized from petroleum ether to obtain a light yellow solid 60.3 grams, with a purity of 98%. 1 H NMR (D 6 -DMSO, 300 MHz): 2.39 (s, 3H), 7.41 (S, 2H), 7.80-7.88 (m, 2H), 8.55 (S, 1H). LC-MS: t R =1.22min, [M+1] + =173.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com