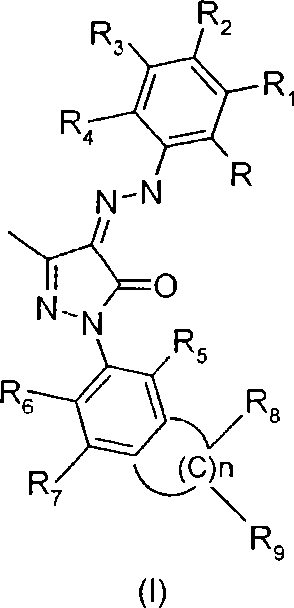
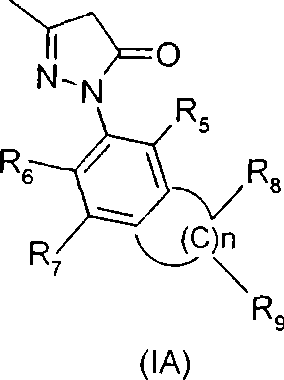
Bicycle substituted pyrazolone azo derivative, preparation thereof and use in medicine
A pharmacy and compound technology, applied in the field of bicyclic substituted pyrazolone azo derivatives, can solve problems such as lack of natural TPO sequence homology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
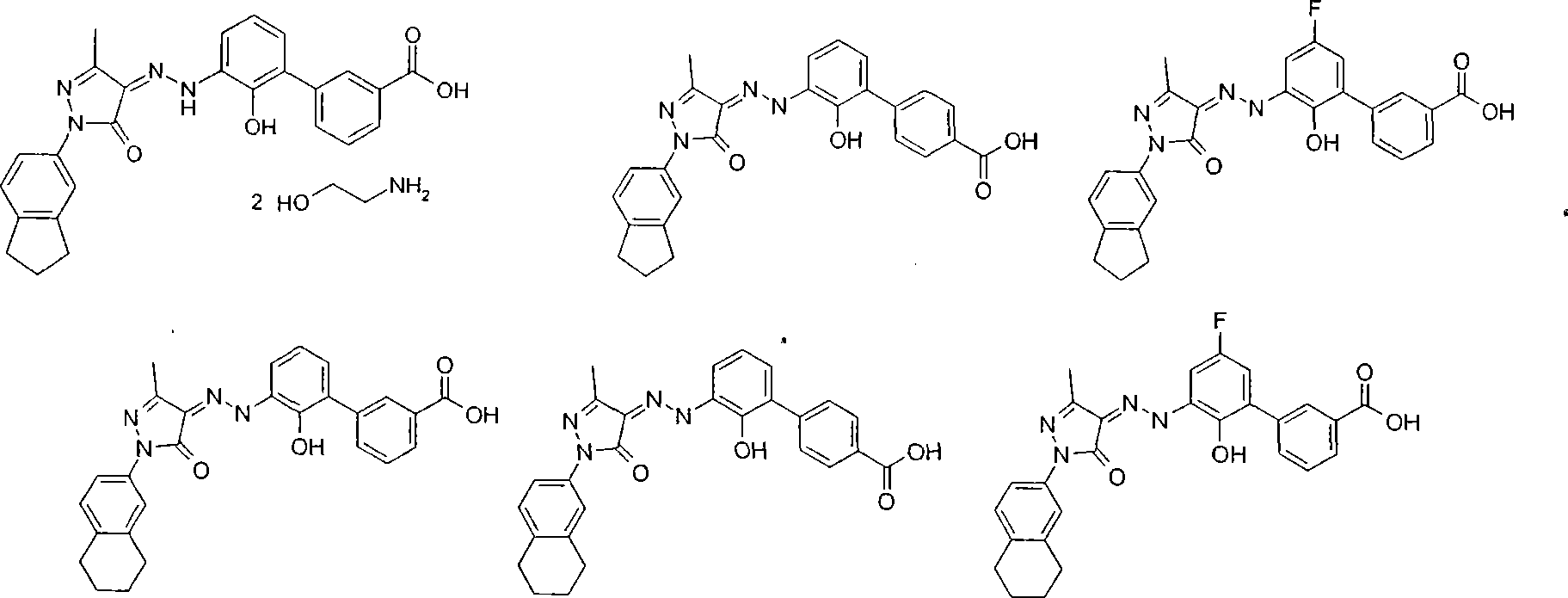
[0091] 2'-Hydroxy-3'-[(2Z)N'-(1-indan-5-yl-3-methyl-5-carbonyl-1,5-dihydro-pyrazole-4-ylidene)- Hydrazino]-biphenyl -3-Carboxylic acid diethanolamine salt
[0092]
[0093] first step
[0094] 2-Bromo-6-nitro-phenol
[0095] Dilute 60 mL of concentrated sulfuric acid into 186 mL of water, add sodium nitrate (79.2 g, 0.932 mol) after cooling to room temperature, keep below 25 °C, add o-bromophenol 1a (60 mL, 0.516 mol) dropwise, and react at room temperature for 2 hours. Track the plate until the raw material disappears, add 320mL ethyl acetate to dissolve the separated solid, wash with water and saturated sodium chloride solution respectively, dry with anhydrous magnesium sulfate, filter, concentrate the filtrate under reduced pressure, and purify the obtained residue by silica gel column chromatography , to give the title product 2-bromo-6-nitro-phenol 1b (48.2 g). Yield: 42.8%.
[0096] MS m / z (ESI): 218 [M+1].
[009...
Embodiment 2
[0142] 2'-Hydroxy-3'-[(2Z)N'-(1-indan-5-yl-3-methyl-5-carbonyl-1,5-dihydro-pyrazole-4-ylidene)- Hydrazino]-biphenyl
[0143] -4-carboxylic acid
[0144]
[0145] first step
[0146] 2′-Methoxy-3′-nitro-biphenyl-4-carboxylic acid
[0147] 1-Bromo-2-methoxy-3-nitro-benzene 1c (23.3g, 0.10mol), 4-carboxyphenylboronic acid (19.5g, 0.117mol) and tetrakis(triphenylphosphine) were mixed under nitrogen atmosphere Palladium (8.86g, 7.7mol) was dissolved in a mixed solvent of 100mL sodium carbonate solution (2N) and 500mL 1,4-dioxane, and heated to reflux at 105°C for 43 hours. Track the plate until the raw materials disappear, concentrate under reduced pressure, add 300mL hydrochloric acid solution (6N) and 400mL ethyl acetate, extract the aqueous phase with ethyl acetate (200mL×2), combine the organic phases, dry with anhydrous magnesium sulfate, filter, The filtrate was concentrated under reduced pressure to give the title product 2'-methoxy-3'-nitro-bip...
Embodiment 3
[0163] 5'-fluoro-2'-hydroxy-3'-[(2Z)N'-(1-indane-5-yl-3-methyl-5-carbonyl-1,5-dihydro-pyrazole-4 -subunit)-hydrazino]-linked
[0164] Phenyl-3-carboxylic acid
[0165]
[0166] first step
[0167] 2-Bromo-4-fluoro-6-nitro-phenol
[0168] 2-Bromo-4-fluoro-phenol 3a (8.0 g, 41.9 mmol) was dissolved in 10 mL of sulfuric acid solution (50%) under ice-salt bath, and sodium nitrate (7.1 g, 83.5 mmol) was added dropwise in 24 mL of sulfuric acid solution ( 25%), reacted at room temperature for 1.5 hours. Spot the trace until the raw material disappeared, add 50mL of water, extract with ethyl acetate (50mL×2), combine the organic phases, wash the ethyl acetate layer with water and saturated sodium bicarbonate solution, dry with anhydrous magnesium sulfate, filter, and the filtrate Concentration under reduced pressure gave the title product 2-bromo-4-fluoro-6-nitro-phenol 3b (8.0 g), which was directly used in the next reaction with a yield o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com