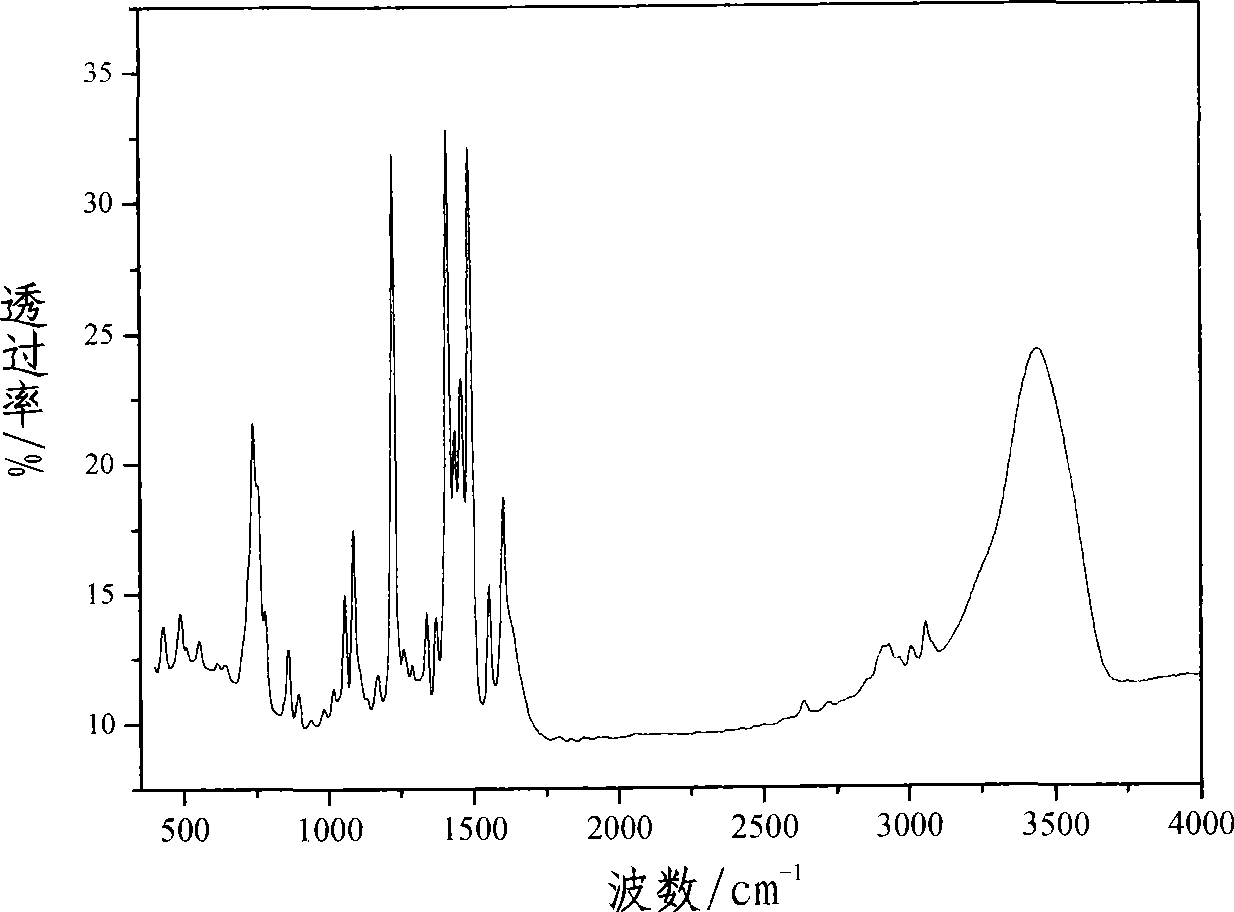
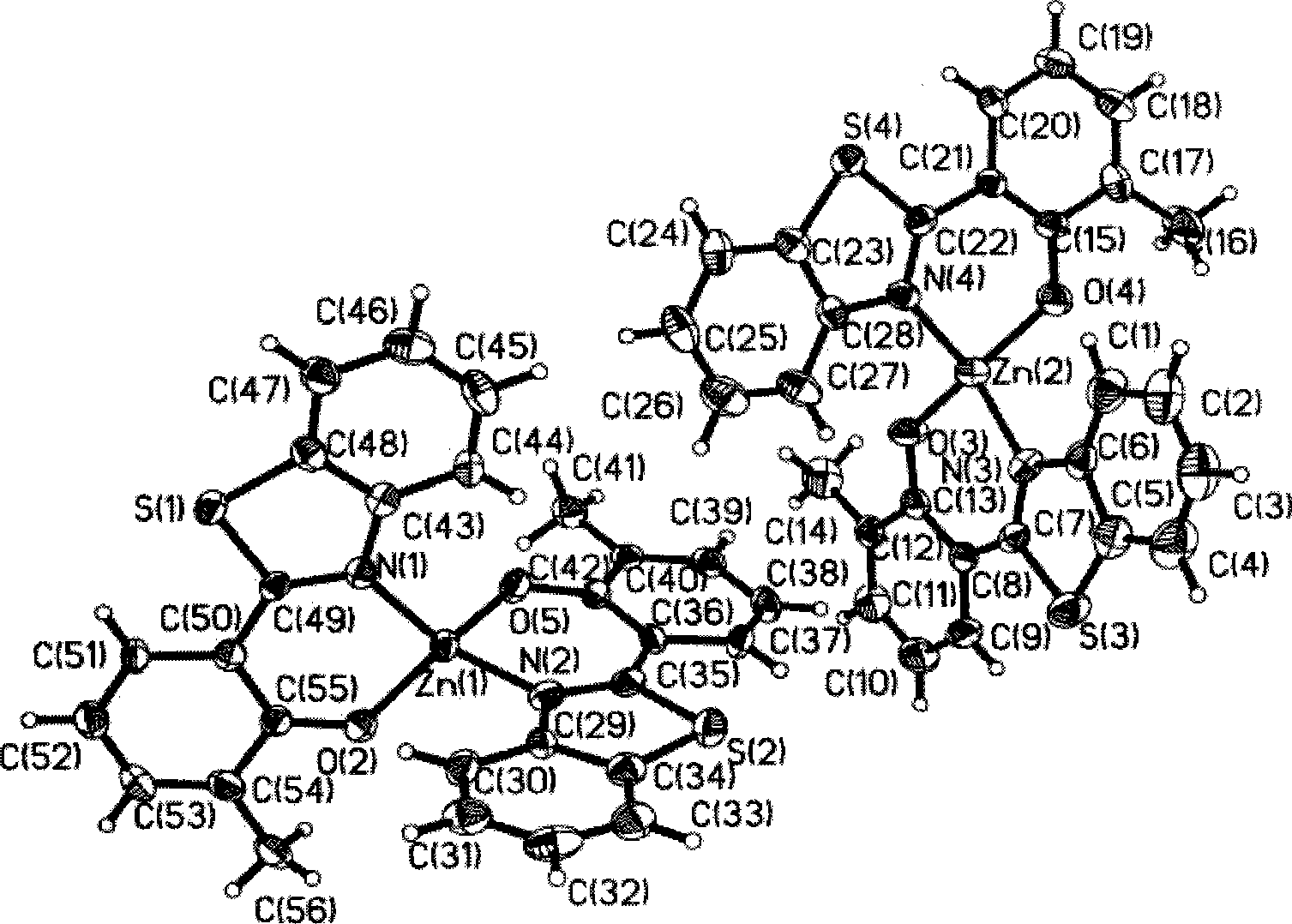
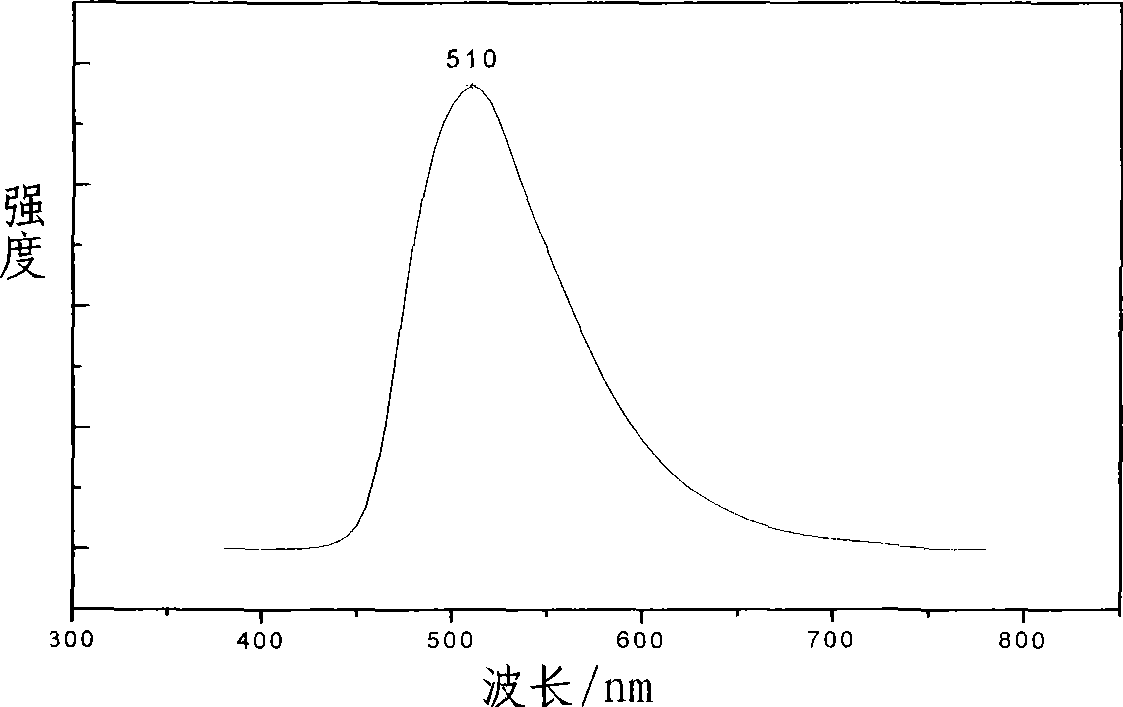
Electroluminescent organic material based on benzothiazolyl
An electroluminescent material, benzothiazolyl-based technology, applied in the direction of luminescent materials, zinc organic compounds, chemical instruments and methods, etc., can solve problems such as substituents affecting luminescence, and other properties without a systematic understanding, to achieve Improvement of electron transport performance, overcoming unbalanced carrier transport, and high thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Add 3.23mL of o-aminothiophenol and 4.56g of 3-methylsalicylic acid into a 250mL three-necked flask with 30mL of toluene, heat to fully dissolve the 3-methylsalicylic acid, stop heating, and slowly add the catalyst phosphorus trichloride dropwise 0.75mL, after the dropwise addition, the system was heated to 80°C for 2.5h under reflux, filtered, and recrystallized with absolute ethanol to obtain colorless crystals of (3-methyl 2-hydroxyphenyl)benzothiazole with a yield of 65 %. The reaction equation is as follows:
[0046]
[0047] Weigh 4.8g (3-methyl 2-hydroxyphenyl) benzothiazole and add it into a 250mL three-necked flask filled with 20mL of anhydrous methanol, heat to dissolve (3-methyl 2-hydroxyphenyl) benzothiazole, slowly Add dropwise 20 mL of anhydrous methanol dissolved in 1.83 g of zinc acetate, react under reflux for 2.5 h, and heat filter to obtain 2-(3-methyl 2-hydroxyphenyl) benzothiazole zinc, abbreviated as Zn(3-MeBTZ) 2 , the reaction equation is as...
Embodiment 2
[0057] Add 3.23mL of o-aminothiophenol and 4.56g of 5-methylsalicylic acid into a 250mL three-necked flask with 30mL of toluene, heat to fully dissolve the 5-methylsalicylic acid, stop heating, and slowly add the catalyst phosphorus trichloride dropwise 0.7mL, after the dropwise addition, the system was heated to 85°C for reflux reaction for 2h, filtered, and recrystallized with absolute ethanol to obtain colorless crystals of (5-methyl 2-hydroxyphenyl)benzothiazole with a yield of 70% . The reaction equation is as follows:
[0058]
[0059] Weigh 3.6g (5-methyl 2-hydroxyphenyl) benzothiazole and add it into a 250mL three-necked flask filled with 20mL of anhydrous methanol, heat to dissolve (5-methyl 2-hydroxyphenyl) benzothiazole, slowly Add 20 mL of anhydrous methanol dissolved with 1.37 g of zinc acetate dropwise, react under reflux for 2 h, and heat filter to obtain 2-(5-methyl 2-hydroxyphenyl) benzothiazole zinc, abbreviated as Zn(5-MeBTZ) 2 , the reaction equation i...
Embodiment 3
[0068] Add 3.23mL of o-aminothiophenol and 6.18g of 4-trifluoromethylsalicylic acid into a 250mL three-necked flask, add 50mL of toluene, heat to fully dissolve 4-trifluoromethylsalicylic acid, stop heating, and slowly add catalyst three Phosphorus chloride 0.8mL, after the dropwise addition, the system was heated to 85°C to reflux for 2h, filtered, and recrystallized with absolute ethanol to obtain colorless crystals of (4-trifluoromethyl 2-hydroxyphenyl)benzothiazole , yield 80%. The reaction equation is as follows:
[0069]
[0070] Weigh 5.9g (4-trifluoromethyl 2-hydroxyphenyl)benzothiazole and add it into a 250mL three-necked flask filled with 30mL of anhydrous methanol, and heat to make (4-trifluoromethyl 2-hydroxyphenyl)benzothiazole Dissolve thiazole, slowly add 20 mL of anhydrous methanol solution with 1.83 g of zinc acetate dropwise, react under reflux for 2 h, and heat filter to obtain 2-(4-trifluoromethyl 2-hydroxyphenyl) benzothiazole zinc, abbreviated as Zn ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com