Mercury resistant chelate monoclonal antibody, as well as preparation method and application thereof
A monoclonal antibody and chelate technology, applied in chemical instruments and methods, instruments, analytical materials, etc., can solve the problems of inability to use on-site detection, limited processing capacity, long detection time, etc., and achieve fast detection speed and selectivity Strong, high titer effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
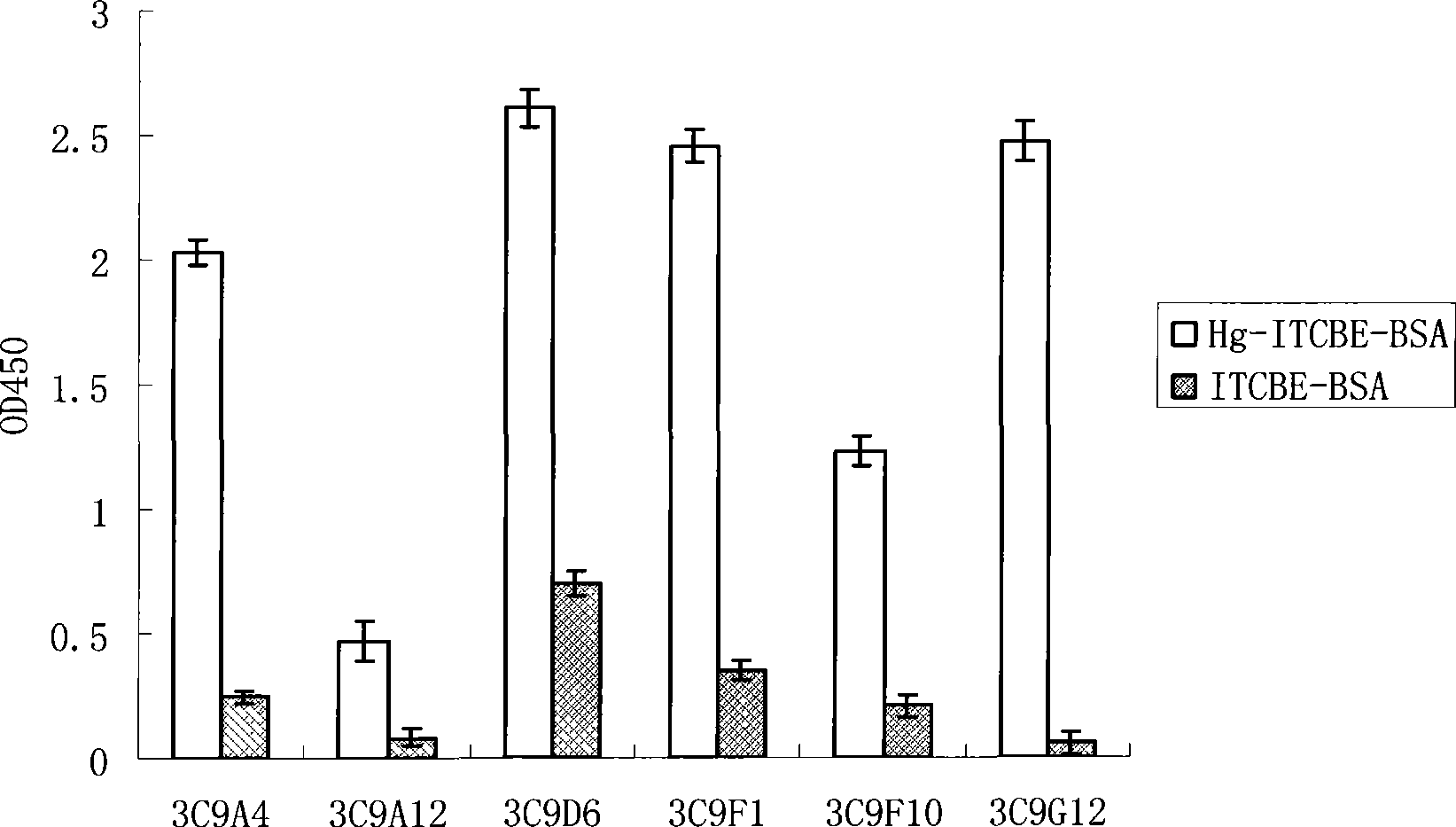
Examples
Embodiment 1
[0039] (1) Dissolve BSA (bovine serum albumin) and KLH (keyhole limpet hemocyanin) in PBS (phosphate buffered saline) at pH 9.5, respectively, with a concentration of 4 mg / mL;
[0040] (2) 10 mg of chelating agent ITCBE was dissolved in 0.1 mol / L, pH 9.5 sodium phosphate buffer solution, the concentration was 18 mg / mL;
[0041] (3) Synthesis of hapten Hg-ITCBE: 1000μL 1mg / mLHg 2+Add the standard solution to 2000 μL of 4.6mM chelating agent solution, adjust the pH to 7.0 with ammonia water, and stir for 12 hours at 20°C;
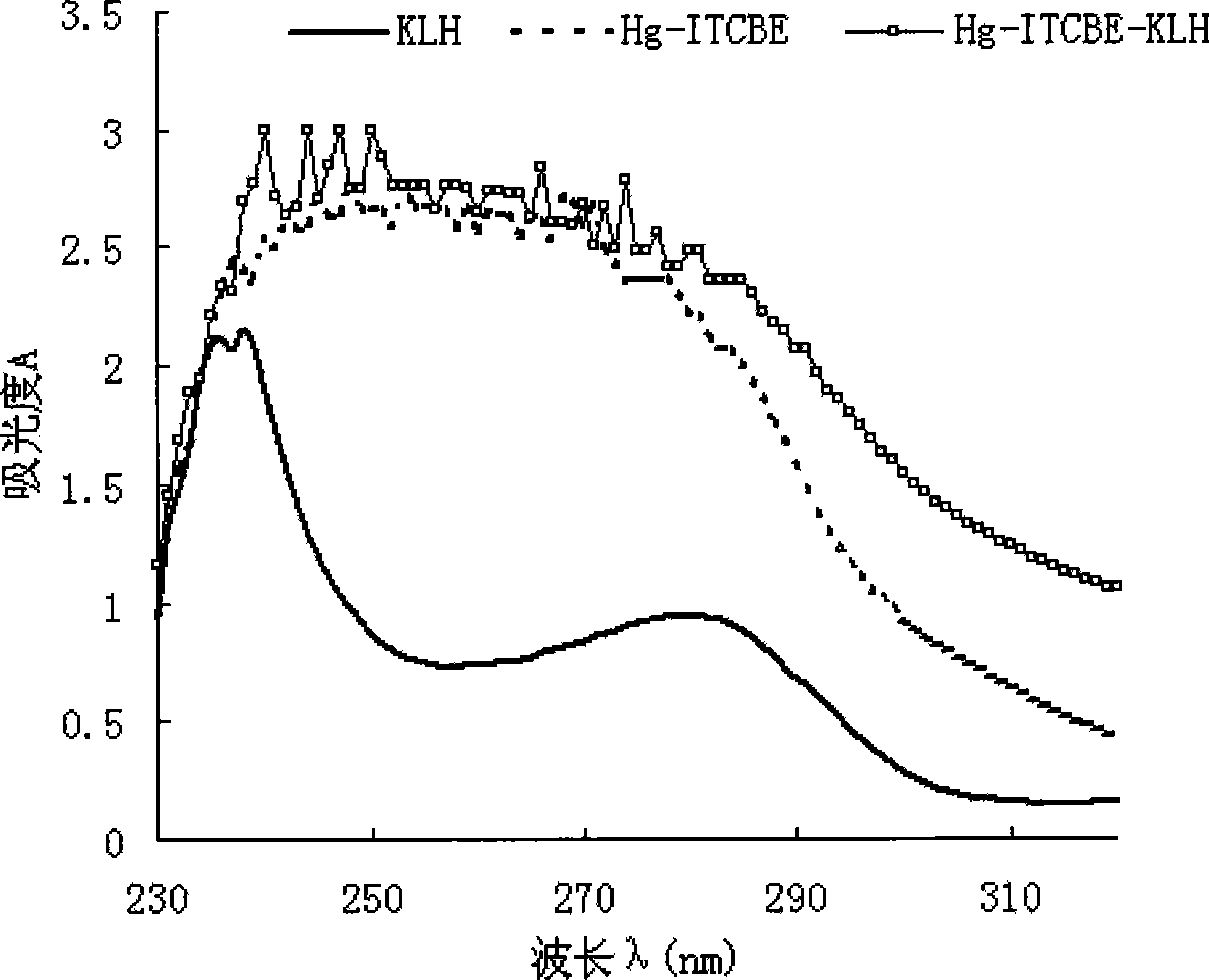
[0042] (4) Synthesis of immunogen Hg-ITCBE-KLH: take 2 mg of hapten, add 2 mg of KLH, adjust the pH to 8.5 with ammonia water, and react with stirring at 20° C. for 12 hours.
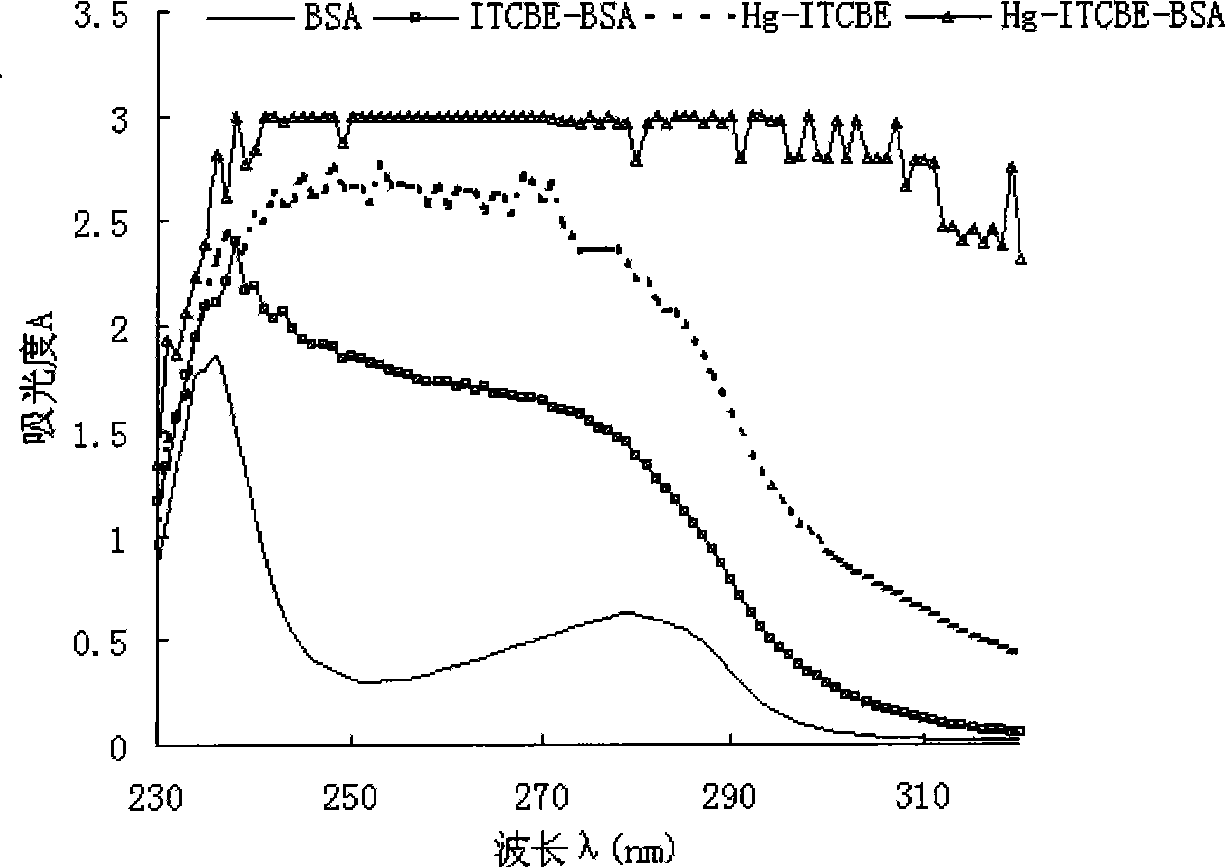
[0043] (5) Synthesis of coated antigen Hg-ITCBE-BSA: take 2 mg of hapten, add 2 mg of BSA, adjust the pH to 8.5 with ammonia water, and react with stirring at 20°C for 12 hours.
[0044] (6) Purification of immunogens and antigens: after the coupling reaction, unreacted small molecul...
Embodiment 2
[0052] (1) Dissolve BSA and KLH in PBS at pH 9.5, respectively, at a concentration of 5 mg / mL.
[0053] (2) Dissolve 10 mg of chelating agent ITCBE in 0.1 mol / L, pH 9.5 sodium phosphate buffer solution with a concentration of 20 mg / mL.
[0054] (3) Synthesis of hapten Hg-ITCBE: 1000μL 1mg / LHg 2+ The standard solution was added to 2000 μL of 4.6 mM chelating agent solution, the pH was adjusted to 7.5 with ammonia water, and the reaction was stirred at 25° C. for 24 h.
[0055] (4) Synthesis of the immunogen Hg-ITCBE-KLH: take 3 mg of hapten, add 3 mg of KLH, adjust the pH to 9.0 with ammonia water, and react with stirring at 25° C. for 24 hours.
[0056] (5) Synthesis of the coated antigen Hg-ITCBE-BSA: take 3 mg of hapten, add 3 mg of BSA, adjust the pH to 9.0 with ammonia water, and react with stirring at 25° C. for 24 hours.
[0057] (6) Purification of immunogens and antigens: After the coupling reaction, unreacted small molecular substances were removed by centrifugation...
Embodiment 3
[0069] (1) Dissolve BSA and KLH in PBS at pH 9.5, respectively, at a concentration of 6 mg / mL.
[0070] (2) Dissolve 10 mg of chelating agent ITCBE in 0.1 mol / L, pH 9.5 sodium phosphate buffer solution with a concentration of 20 mg / mL.
[0071] (3) Synthesis of hapten Hg-ITCBE: 1000μL 1mg / LHg 2+ The standard solution was added to 2000 μL of 4.6 mM chelating agent solution, the pH was adjusted to 7.5 with ammonia water, and the reaction was stirred at 25° C. for 36 h.
[0072] (4) Synthesis of the immunogen Hg-ITCBE-KLH: take 3 mg of hapten, add 3 mg of KLH, adjust the pH to 9.0 with ammonia water, and react at 25° C. for 36 h.
[0073] (5) Synthesis of the coated antigen Hg-ITCBE-BSA: take 3 mg of hapten, add 3 mg of BSA, adjust the pH to 9.0 with ammonia water, and react with stirring at 25°C for 36 hours.
[0074] (6) Purification of immunogens and antigens: after the coupling reaction, unreacted small molecular substances were removed by centrifugation using centeicon-30 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com