Avian influenza vaccine and preparation thereof
A bird flu virus and recombinant gene technology, applied in the field of bird flu vaccine and its preparation, can solve problems such as poor antibody response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0037] Experimental Example 1: Construction of recombinant gene SP-HA-TM.
[0038] The main antigenic gene of avian influenza virus is the HA gene, and the 5' and 3' ends of the HA gene are respectively connected with the signal peptide gene sequence encoding the baculovirus envelope protein gp64 and the transmembrane domain gene sequence encoding the baculovirus envelope protein gp64 , to construct the recombinant gene. By primer design, BamH I and Not I endonuclease restriction sites were introduced at the 5' and 3' ends of the recombinant gene, respectively. Three pairs of primers were designed using the signal peptide gene sequence of baculovirus AcNPV envelope protein gp64, the HA gene sequence of avian influenza virus H5N1, and the transmembrane domain gene sequence of baculovirus AcNPV envelope protein gp64 as templates, and first PCR amplified Target fragments: gp64 signal peptide gene sequence (sp), HA gene and gp64 transmembrane domain gene sequence (tm), and then u...
experiment example 2
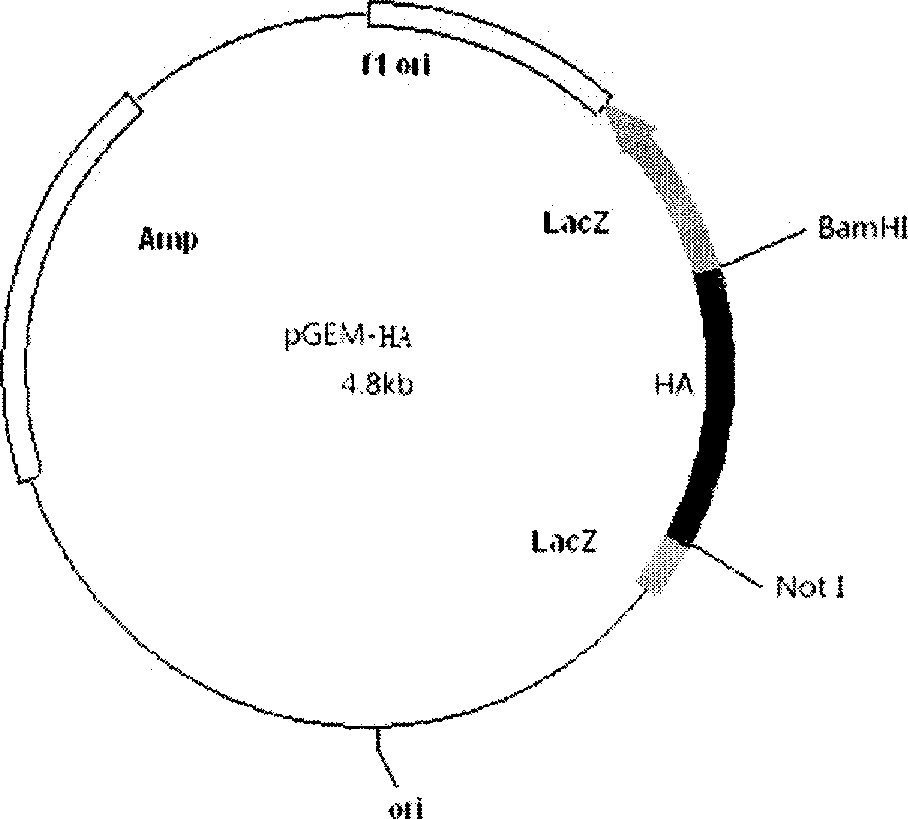
[0098] Experimental Example 2: Construction of Cloning Plasmid pGEM-HA
[0099] Connect the recombinant gene sp-HA-tm amplified by PCR to the pGEM-T vector:
[0100] Recombinant gene sp-HA-tm 2ul
[0101] pGEM-T vector 0.5ul
[0102] 2×Rapid Ligation buffer 5ul
[0103] T4DNA ligase 1ul
[0104] wxya 2 O 1.5ul
[0105] 10ul
[0106] The above components were mixed and reacted at 25°C for 1h.
[0107] The reaction mixture was transformed into competent cells E.coli DH5α, spread on a plate, and cultured at 37°C. Colonies could appear after 12-16 hours. Pick a single colony, extract the plasmid, carry out enzyme digestion and sequencing, the sequence is completely correct, and the plasmid pGEM-HA is obtained.
experiment example 3
[0108] Experimental Example 3: Acquisition of the Baculovirus Transfer Plasmid pBacHA Containing the Recombinant Gene SP-HA-TM
[0109] Plasmid pGEM-HA was digested by BamH I and Not I, and the fragment of recombinant gene SP-HA-TM was cut out, and cloned into the plasmid pBacPAK8 that was digested by BamH I and Not I, and the recombinant gene SP-HA-TM was placed Under the control of the polyhedrin (ph) gene promoter, a recombinant transfer plasmid pBacHA was constructed, and the sequence of the gene was confirmed to be correct by restriction analysis.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum tolerated dose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com