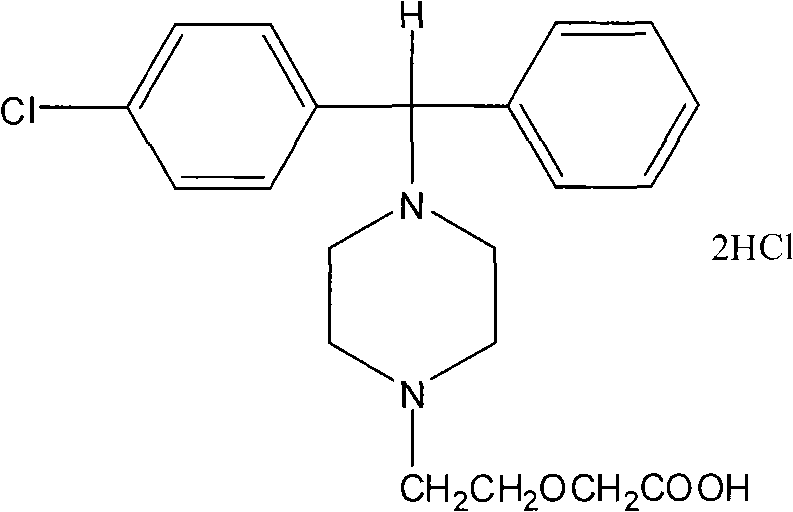
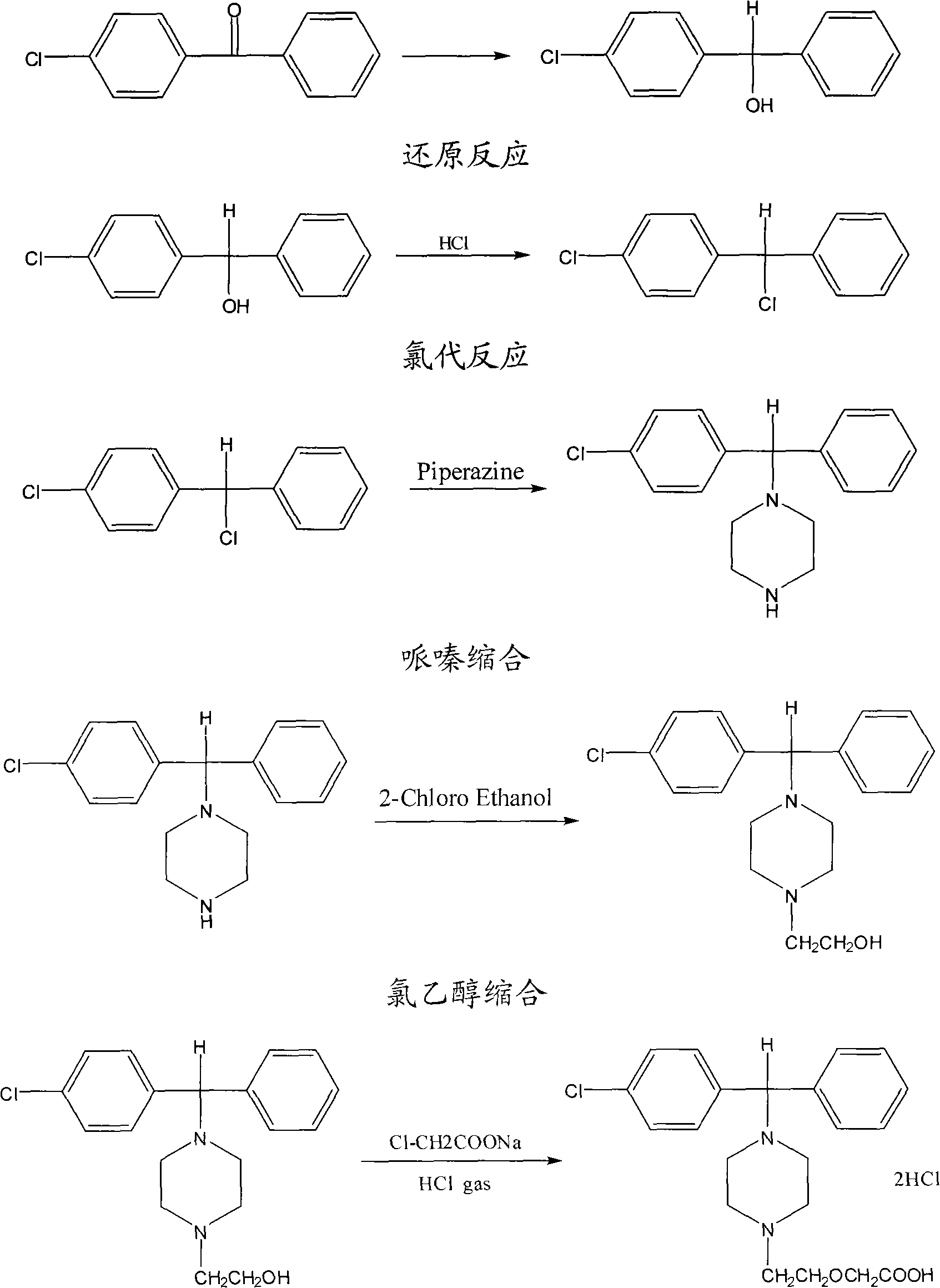
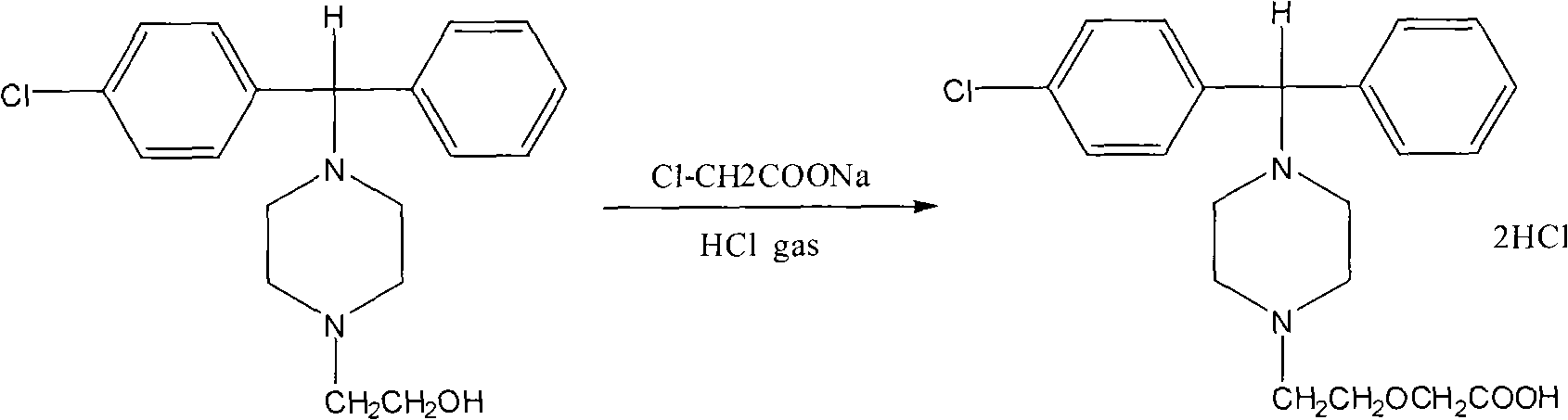
Method for preparing high-purity cetirizine hydrochloride
A kind of cetirizine hydrochloride, cetirizine technology, applied in the field of medicine and chemical industry, can solve the problems of difficult to guarantee product quality, high price of potassium tert-butoxide, no technical advantages, etc., to reduce the cost of production raw materials, reduce the reduction of secondary substitution Response, the effect of reducing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] In a 1000ml four-necked flask, add 100g of 4-[(4-chlorophenyl)phenyl-]1-hydroxyethylpiperazine, then add 175ml of DMSO, stir at room temperature until dissolved, then add 30g of caustic soda After stirring for 1 hour, slowly add 80g of sodium chloroacetate at 10°C to 20°C, finish adding in about 1 hour, and react at 30 to 40°C for 12 hours. TLC detects that the raw materials should be basically reacted completely. After the reaction is complete, add 500ml of water, stir for half an hour, then adjust the pH value to about 9.0 with 10% (w / w) hydrochloric acid, add 200ml of toluene, extract 3 times, and continue to use 10% (w / w) for the water layer. / w) hydrochloric acid to adjust the pH to about 7.5, continue to extract and wash 3 times with 200ml of toluene, continue to use 10% (w / w) hydrochloric acid to adjust the pH to about 4.0 for the aqueous layer, and use 200ml of dichloromethane After extraction three times, the organic layers were combined, and the dichloromethan...
Embodiment 2
[0036] In a 1000ml four-necked flask, add 100g of 4-[(4-chlorophenyl)phenyl-]1-hydroxyethylpiperazine, then add 175ml of DMF, stir at room temperature until dissolved, then add 40g of caustic soda After stirring for 1 hour, slowly add 80g of sodium chloroacetate at 5℃~10℃, finish adding in about 1 hour, and react at 20~30℃ for 12 hours. TLC detection of raw materials should basically complete the reaction. After the reaction is complete, add 500ml of water, stir for half an hour, then adjust the pH to about 9.0 with about 10% hydrochloric acid, add 200ml of ethyl acetate, extract 3 times, and continue to adjust the pH of the water layer with about 10% hydrochloric acid When the pH value is around 7.0, continue to extract and wash 3 times with 200 ml of ethyl acetate, continue to adjust the pH value of the aqueous layer to around 4.0 with about 10% hydrochloric acid, extract three times with 200 ml of dichloromethane, combine the organic layers, and concentrate under reduced pre...
Embodiment 3
[0038] In a 1000ml four-necked flask, add 100g of 4-[(4-chlorophenyl)phenyl-]1-hydroxyethylpiperazine, then add 175ml of DMSO, stir at room temperature until dissolved, then add 40g of hydroxide Potassium, after stirring for 1 hour, slowly add 80g of sodium chloroacetate at 10 ℃ ~ 15 ℃, add in about 1 hour, react at 25 ~ 35 ℃ for 12 hours, TLC detection of raw materials should basically complete the reaction. After the reaction is complete, add 500ml of water, stir for half an hour, then use about 10% hydrochloric acid to adjust the pH to about 9.5, add 200ml of toluene to extract 3 times, and continue to adjust the pH to 7.5 with about 10% hydrochloric acid for the water layer. Continue to extract and wash 3 times with 200ml of toluene, continue to adjust the pH value of the aqueous layer to about 4.0 with about 10% hydrochloric acid, extract 3 times with 200ml of dichloromethane, combine the organic layers, and concentrate the dichloromethane under reduced pressure to dryness...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com