Method for synthesizing lanthanum hexaboride nano powder by solid-phase reaction under low temperature
A technology of lanthanum hexaboride and nanopowder, which is applied in the field of preparation of lanthanum hexaboride materials, can solve the problems of high energy consumption, high temperature requirements, and high cost of raw materials, and achieve low reaction temperature, simple and easy-to-control process, and high product quality. good shape effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: The superfine powder based on LaB6 nano cubes is prepared by reacting lanthanum oxide, magnesium powder, iodine and boric acid.
[0026] Take 0.8g of lanthanum oxide, 2.5g of magnesium powder, 3.7g of iodine, and 1.86g of boric acid into a 25ml special stainless steel reaction kettle, seal it and place it in a resistance crucible boiler. React for half an hour; stop heating, cool the reaction kettle to room temperature naturally; open the kettle, add the obtained product to water, wash with hot dilute hydrochloric acid solution, centrifuge and dry, and obtain the pure phase of LaB 6 powder.
[0027] Cu Kα rays (wavelength λ=1.5418 The scanning step speed is 0.08° / sec) as the diffraction light source for X-ray diffraction analysis of the product.
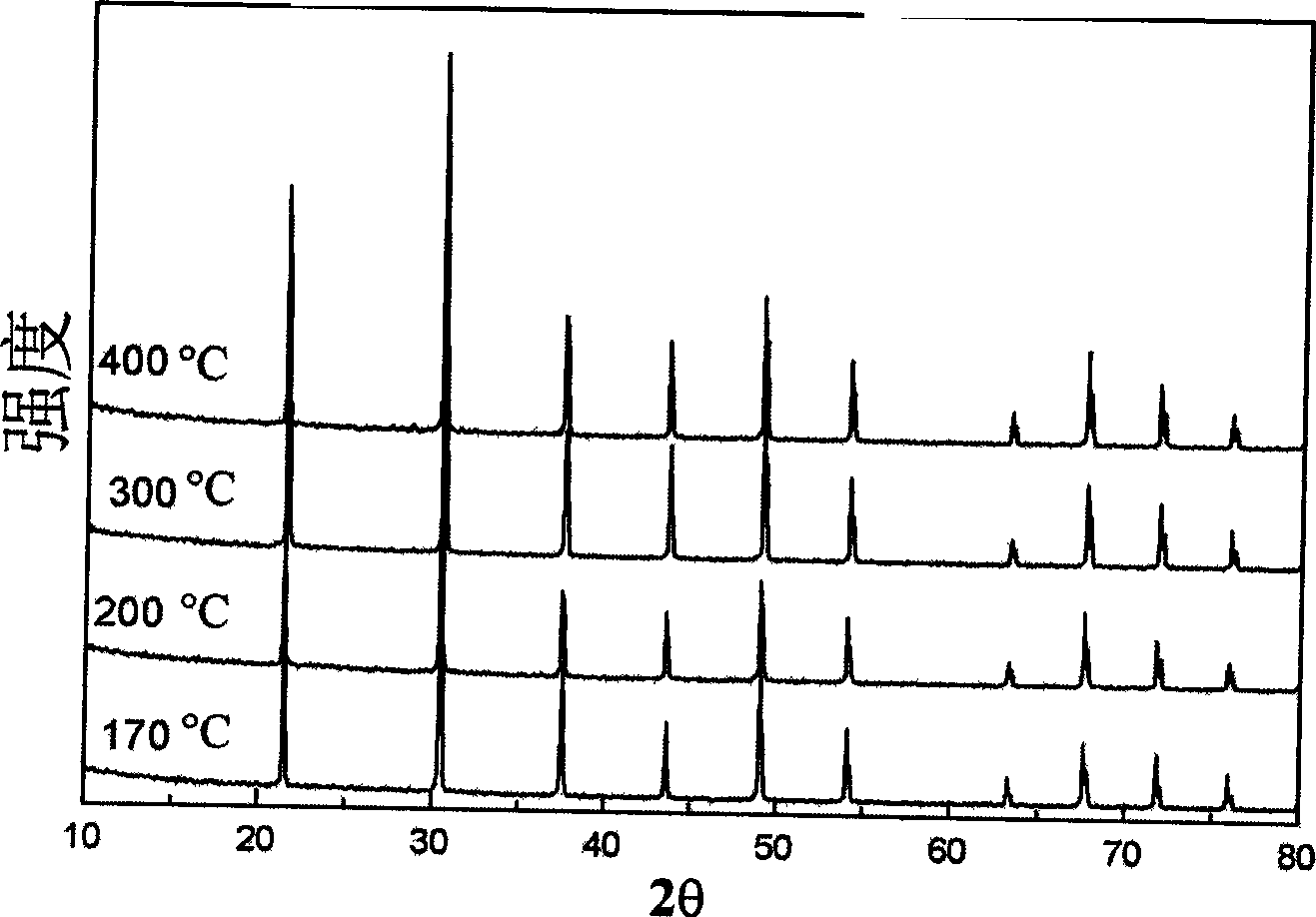
[0028] figure 1 The X-ray diffraction spectrum of the product was prepared by reacting at different temperatures for half an hour with 0.8g lanthanum oxide, 2.5g magnesium powder, 3.7g iodine and 1.86g. Depe...
Embodiment 2
[0030] Example 2: Raw material ratio as described in Example 1, respectively at 170°C, 200°C, 300°C, 400°C, prolonging the reaction time to 12 hours, preparing LaB 6 Ultrafine powder based on nano cubes.
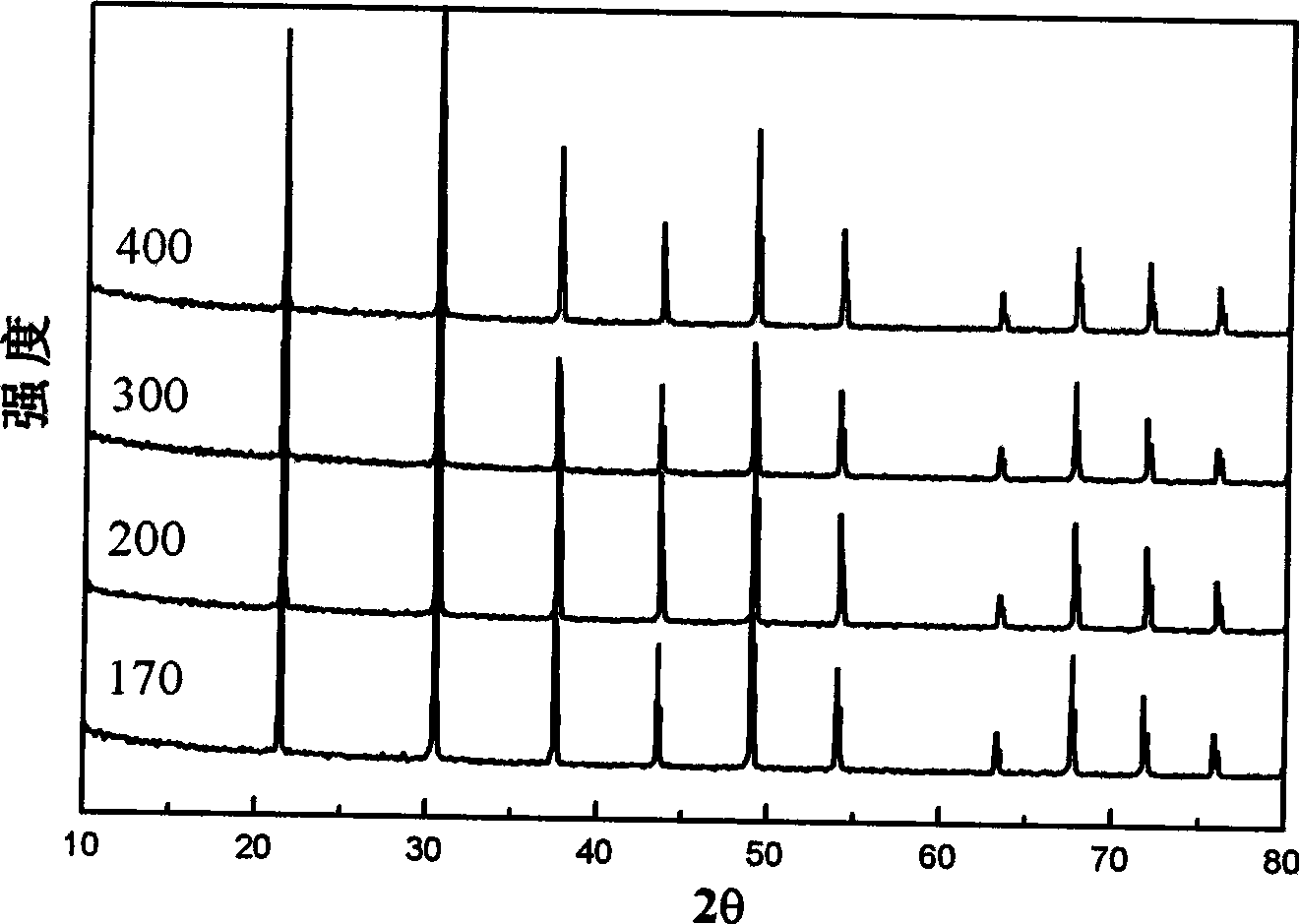
[0031] image 3 The X-ray diffraction spectrum of the product was prepared by reacting 0.8g lanthanum oxide, 2.5g magnesium powder, 3.7g iodine and 1.86g boric acid at different temperatures for 12 hours.
[0032] Depend on image 3 It can be seen that in the X-ray diffraction spectrum, 20 has 10 strong diffraction peaks at 10-80 degrees, and all the diffraction peaks can be indexed as cubic LaB 6 , the position and intensity are consistent with the results of the standard powder diffraction card (JCPDS 34-0427).
[0033] The TEM photo of the product obtained at 170°C is as follows Figure 4 , showing that the nano-LaB6 powder particles are small, and the size is about 300nm.
Embodiment 3
[0034] Embodiment 3: preparation process as described in embodiment 1, difference is that raw material uses 0.3g amorphous boron powder, 1.16g sodium borohydride, 1.0g boron trioxide respectively to replace the boric acid in embodiment 1 as boron source, and Correspondingly adjust the amount of Mg to 0.694g, 0.5g, and 1.0g, respectively, and react at 500°C for 12 hours to prepare LaB 6 Ultrafine powder.
[0035] Figure 5 The X-ray diffraction spectrum of the product was prepared by using amorphous boron powder, sodium borohydride, and boron trioxide as the boron source at 500°C for 12 hours. Depend on Figure 5 It can be seen that all diffraction peaks can be indexed as cubic LaB 6 , the position and intensity are consistent with the results of the standard powder diffraction card (JCPDS 34-0427).
[0036] From boron trioxide (B 2 o 3 ) as a TEM photo of the product obtained as a boron source Figure 6 , showing nano-LaB 6 The powder particles are small and uniform in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com