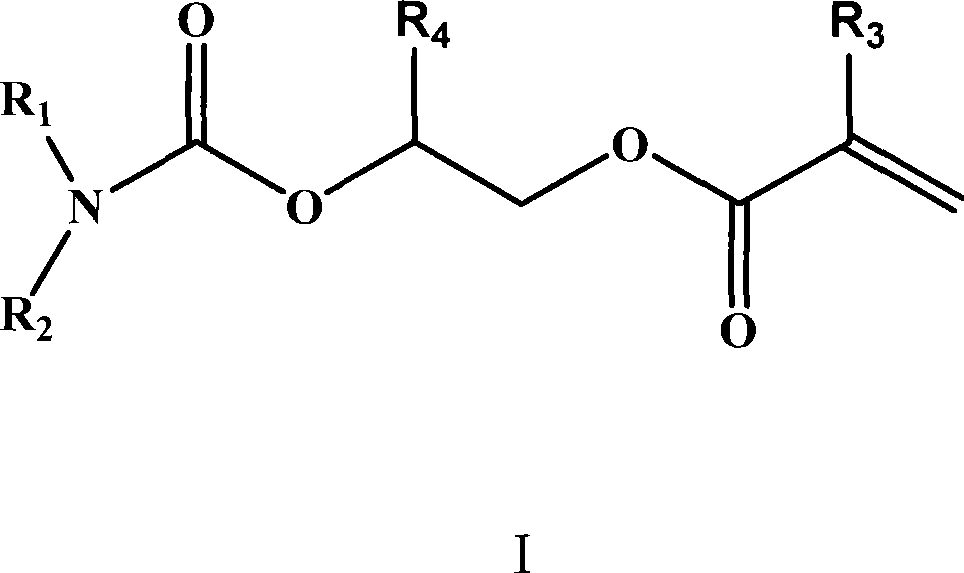
Ultra-low viscosity simple function group carbamate monomer, and preparation and use thereof
A carbamate and monofunctional technology, applied in the field of photosensitive polymer materials, can solve the problems of eye and respiratory tract irritation, irritating odor, high product viscosity, etc., and achieve good dilution ability, less side reactions and high conversion rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
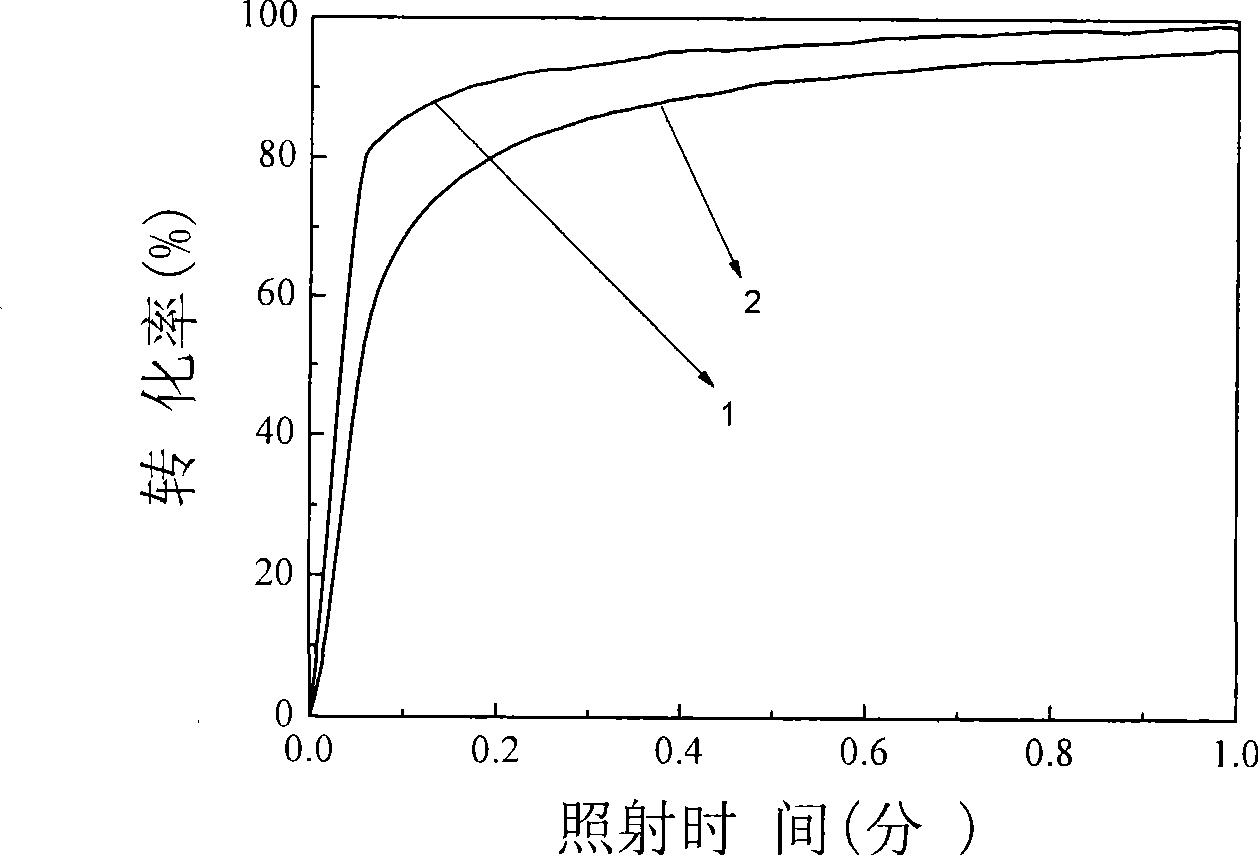
Examples
Embodiment 1
[0027] Preparation and application of 2-(acryloyloxy)ethyl-dicyclohexyl carbamate
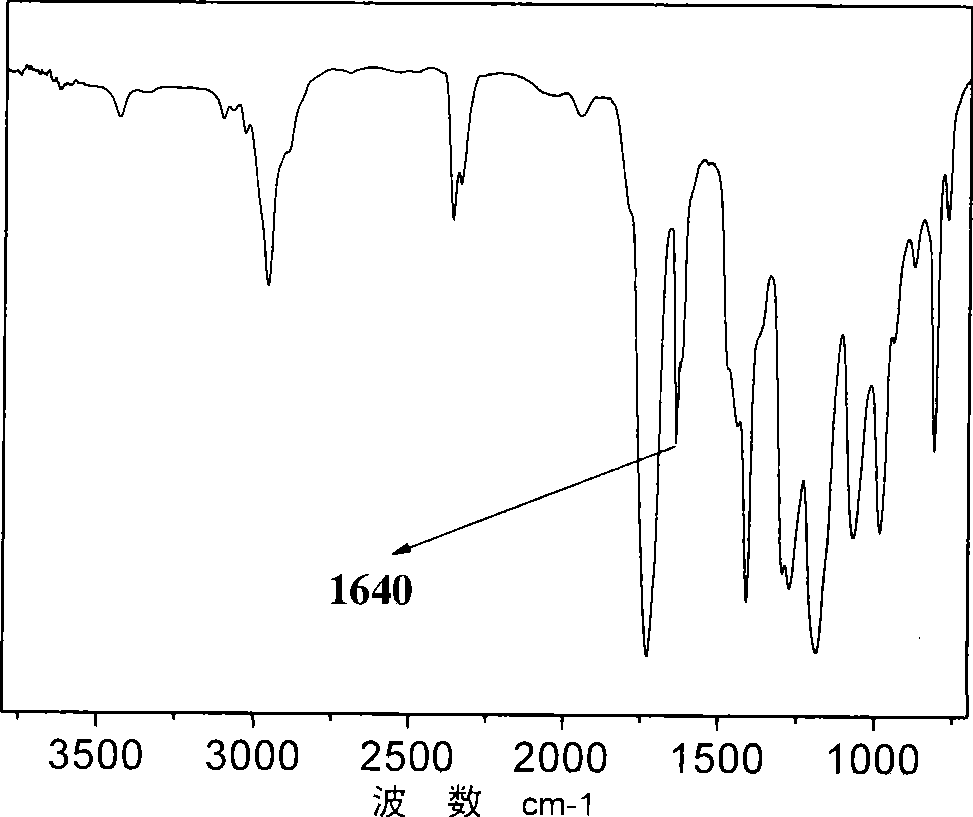
[0028] 1) Weigh 8.81g (0.1mol) of ethylene carbonate and dissolve it in 50ml of toluene, then add 18.13g (0.1mol) of dicyclohexylamine, heat to 90°C and stir at a constant temperature. After 5 hours of reaction, 1803cm in the infrared spectrum -1 The absorption peaks at the left and right disappeared, the reaction was stopped, and the solvent toluene was removed to obtain a colorless transparent sticky substance;
[0029] 2) After adding 27.12g of colorless transparent viscous material, 20g of triethylamine and 100ml of dichloromethane into a three-necked flask with a mechanical stirring device, the reaction temperature is -10°C, and 8.5ml of acryloyl chloride is measured. Drop it into a three-necked flask within 1 hour. After the dropwise addition, continue to stir for 2 hours, let it stand overnight, and filter with suction to obtain a dark red liquid. 3 After washing the dark red liquid wit...
Embodiment 2
[0035] Preparation and application of 2-(acryloyloxy)ethyl-diisopropyl carbamate
[0036] 1) Weigh 8.81g (0.1mol) of ethylene carbonate and dissolve it in 50ml of toluene, then add 10.12g (0.1mol) of diisopropylamine, heat to 30°C and stir at a constant temperature. After 10 hours of reaction, 1803cm in the infrared spectrum -1 The absorption peaks at the left and right disappeared, the reaction was stopped, and the solvent toluene was removed to obtain a colorless transparent sticky substance;
[0037] 2) After adding 18.93g of colorless and transparent viscous matter, 22g of tripropylamine and 100ml of ethyl acetate into a three-necked flask with a mechanical stirring device, the reaction temperature was 0°C, and 8.5ml of acryloyl chloride was After the dropwise addition, continue to stir for 2 hours, let it stand overnight, and filter with suction to obtain a dark red liquid, wash the dark red liquid with dilute hydrochloric acid solution (1M), NaOH solution and distilled w...
Embodiment 3
[0043] Preparation and application of 2-(acryloyloxy)ethyl-N,N-(methyl,phenyl)carbamate
[0044]1) Weigh 8.81g (0.1mol) of ethylene carbonate and dissolve it in 50ml of toluene, then add 10.72g (0.1mol) of N-methylaniline, heat to 120°C and stir at a constant temperature. After reacting for 1 hour, 1803cm in the infrared spectrum -1 The absorption peaks at the left and right disappeared, the reaction was stopped, and the solvent toluene was removed to obtain a colorless transparent sticky substance;
[0045] 2) After adding 19.53g of colorless and transparent viscous matter, 25g of tri-n-butylamine and 100ml of n-hexane into a three-necked flask with a mechanical stirring device, the reaction temperature was 10°C, and 8.5ml of acryloyl chloride was measured. Drop it into a three-necked flask within 1 hour. After the dropwise addition, continue to stir for 2 hours, let stand overnight, and filter with suction to obtain a dark red liquid. Use dilute hydrochloric acid solution (1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com