Biodegradable in-situ solidification sustained-release injector
A slow-release injection, in-situ curing technology, applied in the direction of drug delivery, active ingredients of heterocyclic compounds, and medical preparations of non-active ingredients, etc., can solve the problem of low tolerance, uneven drug distribution, and release batch differences. major problems, to achieve the effect of easy acceptance and good clinical application potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Study on the Technical Route and Quality of PDLLA-mPEG2000 Synthesis
[0043] 1.1 Synthesis, structural analysis and quality research of PDLLA-mPEG2000: The PDLLA-mPEG2000 (injection) studied by our research group has submitted an application for registration of new pharmaceutical excipients to the State Food and Drug Administration. Therefore, the synthesis, structural analysis of the polymer and quality studies will be conducted with reference to it.
[0044] Synthesis: PDLLA-mPEG 2000 98 / 2, 95 / 5, 90 / 10, 80 / 20 and 70 / 30 were synthesized by ring-opening polymerization method. That is: weigh methyl polyethylene glycol and lactide in proportion, place them in a closed reactor, raise the temperature to 120-140°C under nitrogen flow to melt the solid, add 0.5% stannous octoate, and raise the temperature to React at 150-180°C for 3-6 hours. After cooling, a white solid crude product was obtained. After the crude product is dissolved in an appropriate amount of dichlorome...
Embodiment 2
[0049] Preparation and in vitro release study of tinidazole in-situ solidified sustained-release injection
[0050] Accurately weigh a certain proportion of polymer and drug tinidazole powder according to the prescription and place it in a 10mL centrifuge tube, add a certain volume of NMP, and vortex for 30 minutes to form a transparent and uniform solution. That is, the in-situ solidified slow-release injection of tinidazole is obtained.
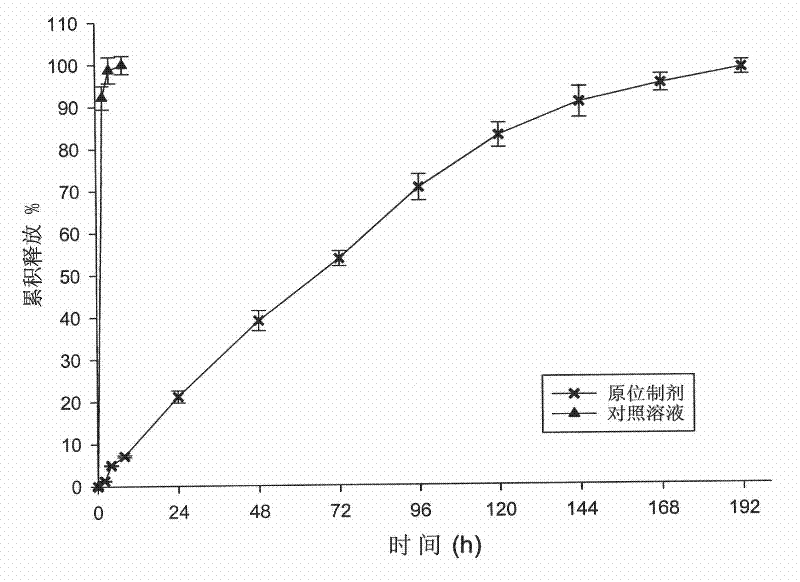
[0051] In vitro release test: Tinidazole in-situ solidified sustained-release injection was prepared, placed in a 10 mL centrifuge tube, and 5 mL of 0.1 mol / L, pH 7.4 PBS buffer solution at 37°C was added to solidify the preparation in situ. Put it into a constant temperature shaker to vibrate at a speed of 100 rpm, and keep the temperature at 37°C. Take 1.5mL release solution 2, 4, 6, 8, and 12 hours after in-situ solidification, and quickly add 1.5mL blank PBS buffer solution, and then take samples every 24 hours until the 8th day. Take...
Embodiment 3
[0054] Preparation and in vitro release study of leuprolide acetate in situ solidified sustained-release injection
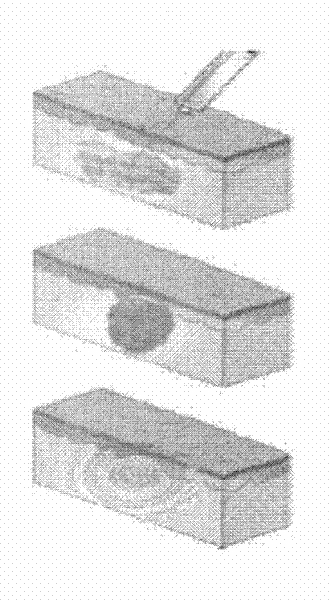
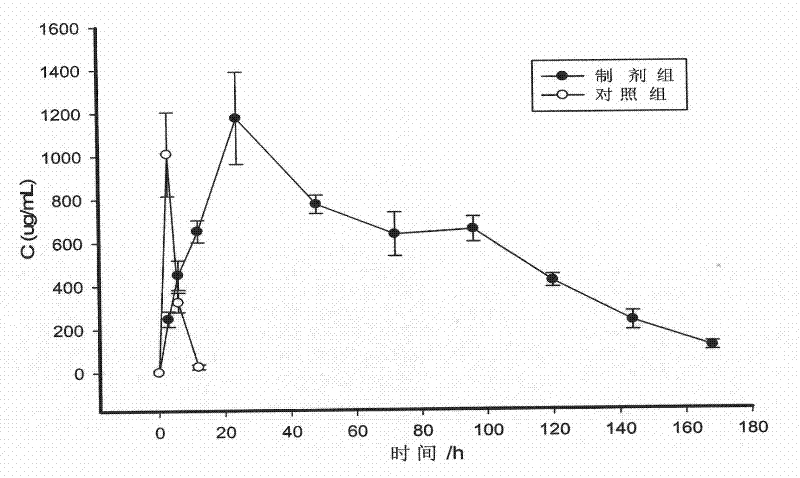
[0055] To prepare leuprolide acetate in-situ solidified sustained-release injection that can be sustained-released for 1 month, the polymer PDLLA-mPEG was dissolved in the organic solvent NMP, vortexed and mixed until the state of a clear and transparent solution was put into syringe A, and Put 7.5mg of leuprolide acetate into syringe B, and sterilize it with gamma-ray radiation. Connect the two syringes before use, and push the piston back and forth until the drug and polymer solution are fully mixed, showing a viscous suspension state. When using, push the preparation completely into one of the syringes, remove the other syringe and connect the injection needle, and inject the preparation under the patient's skin. Take 500 μL of the injection and inject it into the back of the SD rat, and kill it 12 hours later, cut the injection site in the back, and observe ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com