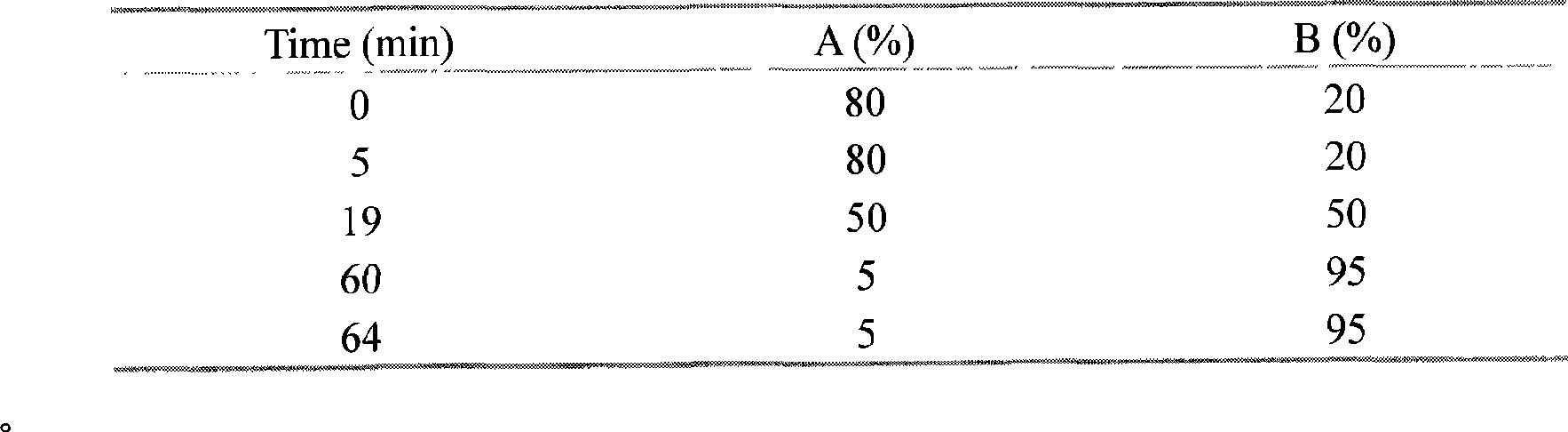Active components of gallnut and preparation method and use thereof
An effective component, the gallnut technology, applied in the direction of drug combination, medical raw materials derived from arthropods, anti-tumor drugs, etc., can solve problems involving normal cells, side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Embodiment 1 The preparation of Galla gall effective components
[0077] Take 250g of Galla gall medicinal material, crush it, add ethyl acetate and ethanol (1:1), heat to reflux for 1 hour, extract twice, and combine the filtrates to obtain the extract. The extract is concentrated into an extract, dissolved and loaded with ethanol, and passed through an ODS-C18 column. At first, 5% ethanol (5BV; 1BV≈250ml) is used as the mobile phase to obtain eluent I, and then 95% ethanol ( 5BV) as the mobile phase, to obtain eluent II, and to obtain a sample after concentrating and drying the eluent II; continue to separate the sample obtained by preparative liquid chromatography; separation conditions for preparative chromatography: the chromatographic column is a preparative column Zorbax SB-C18; 21.2mm×250mm, the mobile phase is water A and acetonitrile B, the gradient elution procedure is as follows:
[0078]
[0079] The flow rate is 10ml / min, and the column temperature is ...
Embodiment 2
[0080] Embodiment 2 The analysis of Galla gall effective components
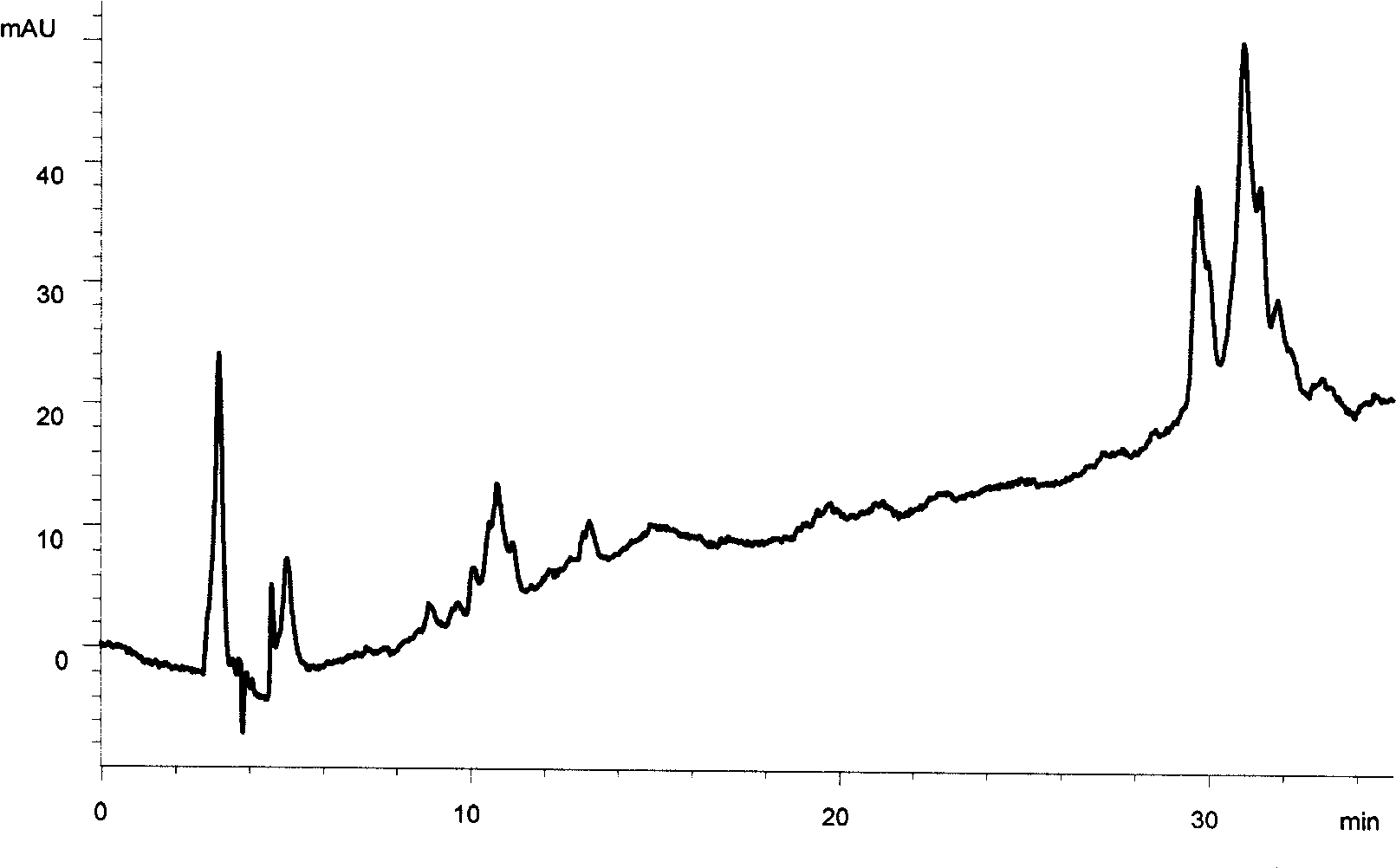
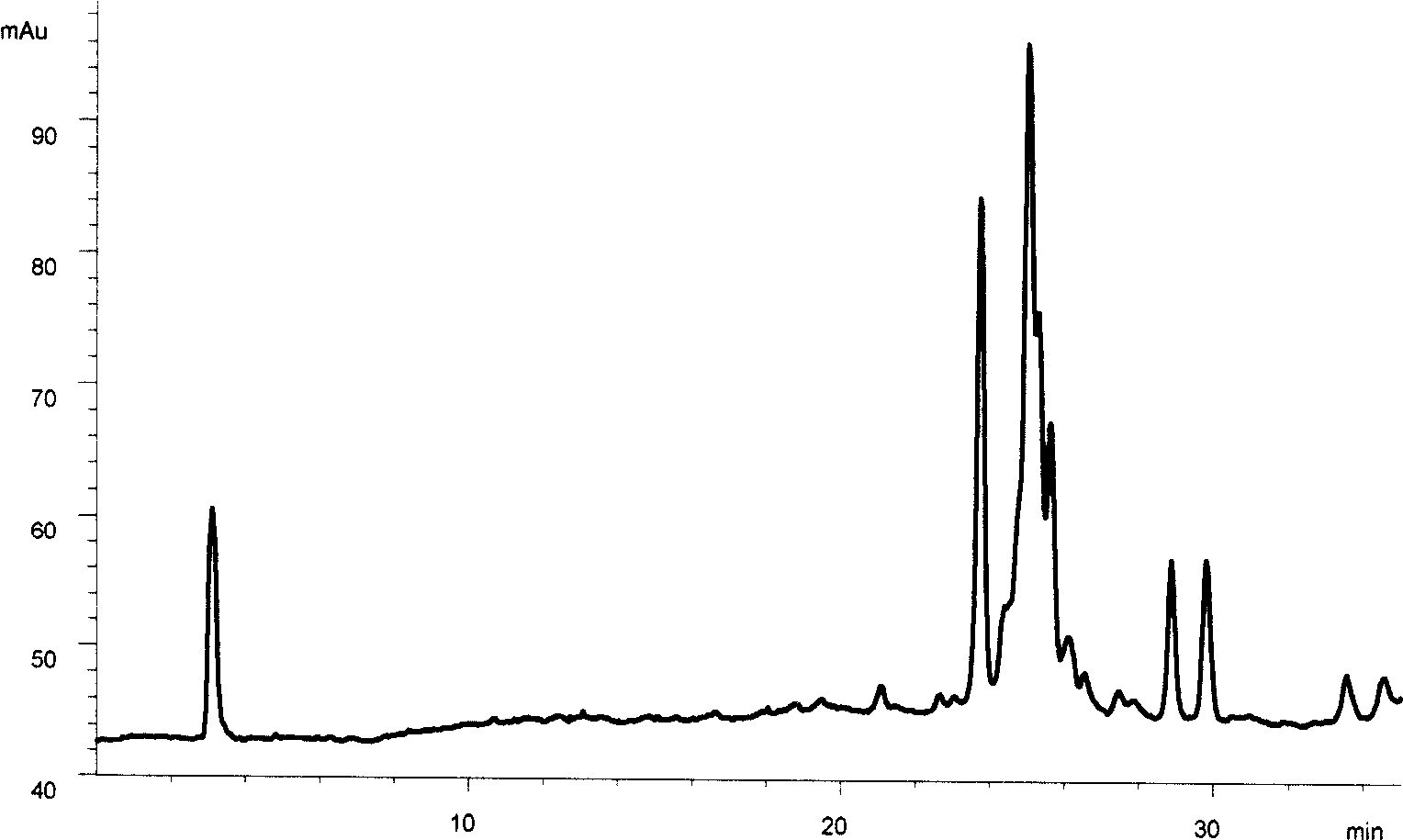
[0081] The HPLC-ELSD joint analysis to the Galla gall effective component of embodiment 1
[0082] Chromatographic conditions Chromatographic column Agilent Zorbax SB-C18 column (4.6mm × 150mm, 5 μm); using gradient elution, mobile phase A is 0.2% glacial acetic acid aqueous solution, mobile phase B is acetonitrile solution containing 0.2% glacial acetic acid; gradient elution The procedure is as follows: at 0 minutes, mobile phase A is 90% 0.2% glacial acetic acid aqueous solution, mobile phase B is 10% 0.2% glacial acetic acid in acetonitrile solution; at 10 minutes, mobile phase A is 50% 0.2% glacial acetic acid Aqueous solution, mobile phase B is 50% 0.2% glacial acetic acid acetonitrile solution; 30 minutes, mobile phase A is 5% 0.2% glacial acetic acid aqueous solution, mobile phase B is 95% 0.2% glacial acetic acid acetonitrile solution; 35 In minutes, the mobile phase A was 5% 0.2% glacial acetic...
Embodiment 3
[0085] Example 3 Galla gall effective component preparation
[0086] Take the gallnut active ingredient of Example 1, mix 0.5g with 10.5g polyethylene glycol-6000 evenly, heat and melt, move the material to the drip irrigation after the material, drop the medicine liquid into the liquid paraffin at 6-8°C, remove Oil, made 400 drop pills.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com