Solvent extraction of ferronickel powder and waste liquor processing method
A treatment method and technology of ferronickel powder, applied in the treatment field of acid leaching and nickel precipitation wastewater, can solve problems such as environmental pollution, leaching agent sulfuric acid cannot be recycled, etc., and achieve the effects of reducing cost, increasing value, and improving utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
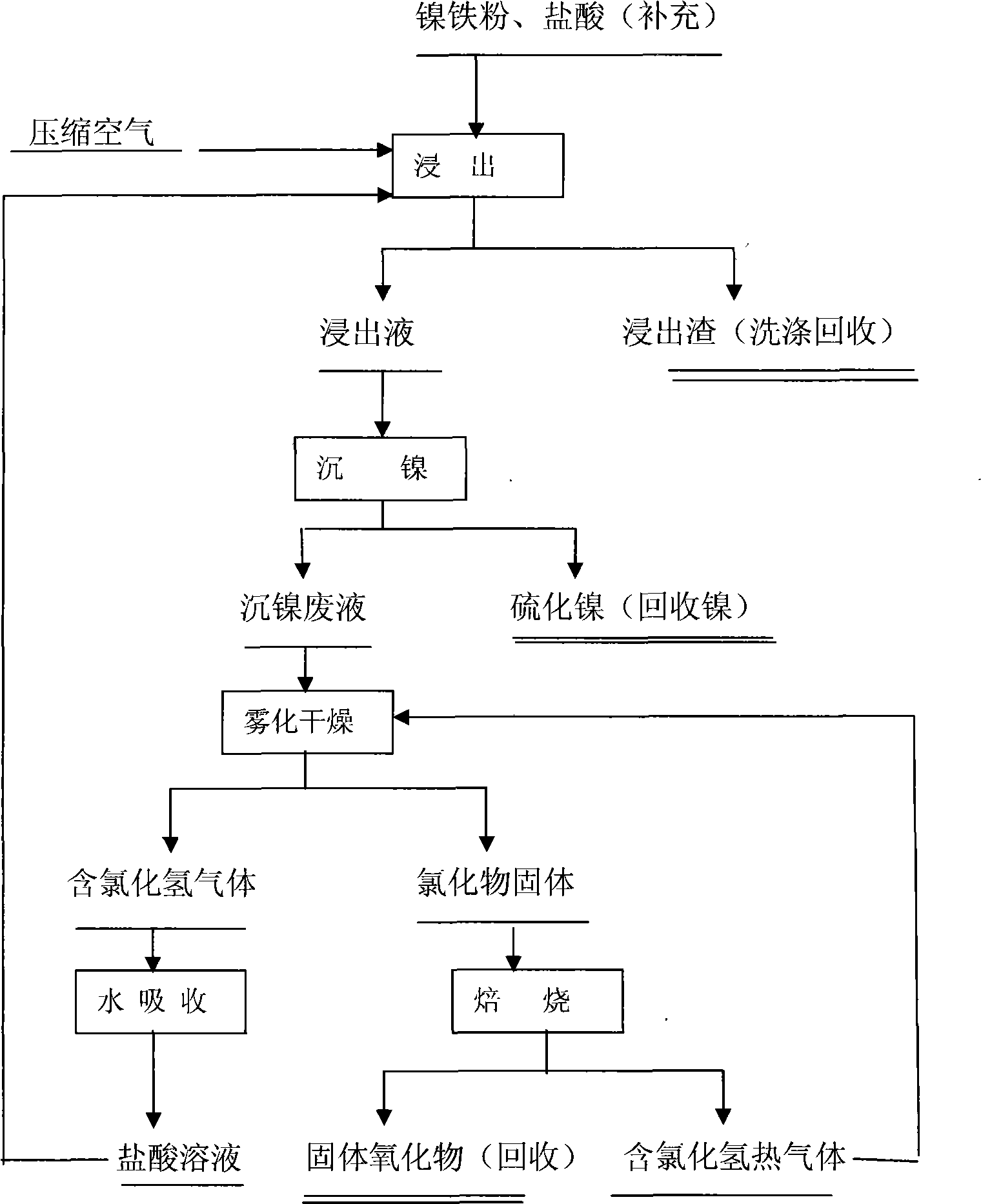
[0020] Main components of nickel iron powder: Ni6.95%, Fe 85.00%, particle size -200 mesh.
[0021] Hydrochloric acid leaching: Put 150Kg ferronickel powder, 980L water and 254Kg hydrochloric acid (containing 31% HCl) into the reactor, the liquid-solid ratio is 8:1, and the amount of hydrochloric acid added is 6 times the theoretical requirement of nickel in the ferronickel powder. Heat to 50-60°C, pass through compressed air, keep stirring and leaching for 18 hours. 152Kg of leaching residue and 1350L of leaching liquid were obtained. The leaching slag contains 0.15% nickel and 60.12% iron. The leaching rate of nickel and iron in the raw material is 97.81% and 28.33%.
[0022] Nickel precipitation in the leaching solution produces 34.40Kg of nickel sulfide, the nickel sulfide contains 28.73% nickel, and the nickel recovery rate is 94.83%.
[0023] The output of nickel precipitation wastewater 13101, the main component is: FeCl 2 87.31g / l, NiCl 2 0.53g / l, pH 2.5-3. 400l o...
Embodiment 2
[0025] Main components of nickel iron powder: Ni6.95%, Fe 85.00%, particle size -200 mesh.
[0026] Hydrochloric acid leaching: put 150Kg ferronickel powder, 600L water and 170Kg hydrochloric acid (containing HCl31%) into the reactor, the liquid-solid ratio is 5:1, the amount of hydrochloric acid added is 4 times the theoretical requirement of nickel in the ferronickel powder, and heated to 70~80℃, pass through the compressed air to keep stirring and leaching for 12 hours. 163Kg of leaching residue and 810L of leaching solution were obtained. The leaching slag contains 0.182% nickel and 64.03% iron. The leaching rate of nickel and iron in the raw material is 97.15% and 18.13%.
[0027] Nickel precipitation in the leaching solution produces 29.80Kg of nickel sulfide, the nickel sulfide contains 32.53% nickel, and the nickel recovery rate is 93.07%.
[0028] Output 780l of nickel precipitation wastewater, the main component is: FeCl 2 105.02g / l, NiCl 2 1.21g / l, pH 2.5-3. 40...
Embodiment 3
[0030] Main components of nickel iron powder: Ni6.95%, Fe 85.00%, particle size -200 mesh.
[0031] Hydrochloric acid leaching: put 150Kg ferronickel powder, 380L water and 85Kg hydrochloric acid (containing HCl31%) into the reactor, the liquid-solid ratio is 3:1, the amount of hydrochloric acid added is twice the theoretical requirement of nickel in the ferronickel powder, and heated to 90~100℃, pass through the compressed air to keep stirring and leaching for 7 hours. 186Kg of leaching residue and 520L of leaching solution were obtained. The leaching slag contains 0.28% nickel and 64.22% iron, and the leaching rate of nickel and iron in the raw material is 95.02% and 6.32%.
[0032] Nickel precipitation in the leaching solution produces 39.81Kg of nickel sulfide, the nickel sulfide contains 24..53% of nickel, and the recovery rate of nickel is 93.67%.
[0033] Output 490l of nickel precipitation wastewater, the main component is: FeCl 2 88.17g / l, NiCl 20.64g / l, pH 2.5-3....
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com