Method for preparing silicon stannum alloy cathode material of lithium ion battery
A lithium-ion battery and alloy negative electrode technology, applied in electrode manufacturing, battery electrodes, circuits, etc., can solve the problems of poor battery cycle, volume expansion of alloy negative electrode materials, etc., achieve high specific energy, reduce production energy consumption, and simple process easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
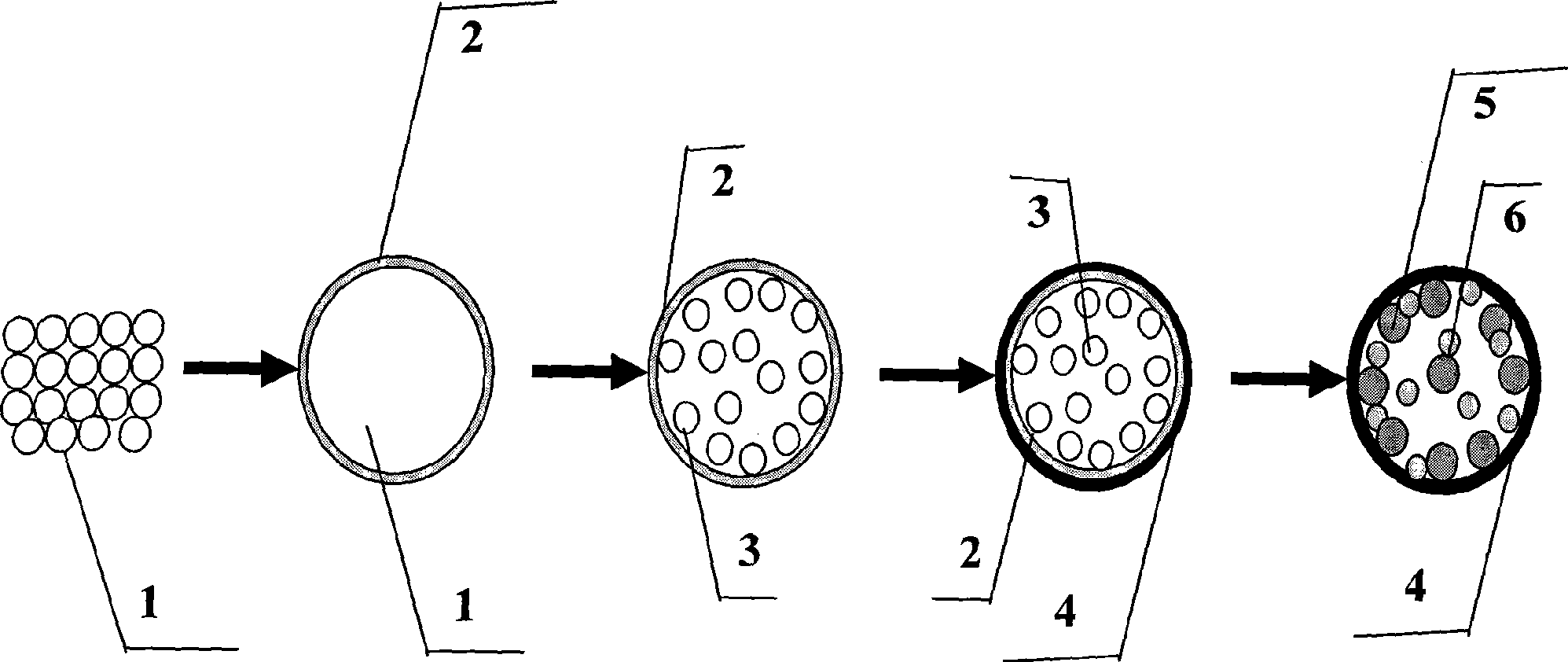
[0042] (1) Take 5mL with a concentration of 1.0mol L -1 Ammonia solution, 100mL methanol and 20mL water were mixed and stirred for 0.5h, 6.25mL methyl silicate was added to the mixture and stirred for 3h, the resulting suspension was centrifuged, washed with deionized water, and dried to obtain silica balls.
[0043] (2) 11.46g urea and 1.7g Na 2 SnO 3 ·3H 2 O was dissolved in 200 mL of deionized water, then 18 mL of ethanol was added thereto, stirred, and a milky suspension was obtained. 3.82 g of the product of step (1) was ultrasonically dispersed in 4 mL of deionized water for 0.5 h at a molar ratio of Sn:Si:urea=1:10:30 to obtain a suspension. The mixture obtained after mixing the two suspensions was transferred to a 300mL high-pressure furnace, heated at 2 atmospheres and 100°C for 2h, cooled, centrifuged, washed, and dried. Take 240mg of the dried product, and 1.3ml, 2mol / L KOH solution in molar ratio (Sn+Si):OH - =1:2, reacted for 2 hours, corroded 60% of the prod...
Embodiment 2
[0047] (1) Take 5mL with a concentration of 1.0mol L -1 Ammonia solution, 100mL ethanol and 20mL water were mixed and stirred for 0.5h, 5mL methyl silicate was added to the mixture and stirred for 10h, the obtained suspension was centrifuged, washed with deionized water, and dried to obtain silica balls.
[0048] (2) 11.46g urea and 1.7g Na 2 SnO 3 ·3H 2 O was dissolved in 200 mL of deionized water, then 18 mL of ethanol was added thereto, stirred, and a milky suspension was obtained. 3.82 g of the product of step (1) was ultrasonically dispersed in 4 mL of deionized water for 1 h at a molar ratio of Sn: Si: urea = 1:10:30 to obtain a suspension. The mixture obtained after mixing the two suspensions was transferred to a 300mL high-pressure furnace, heated at 3 atmospheres and 500°C for 12h, cooled, centrifuged, washed and dried. Take 240mg of the dried product, and 1.3ml, 2mol / L KOH solution in molar ratio (Sn+Si):OH - =1:2, reacted for 10 hours, corroded 60% of the produ...
Embodiment 3
[0052] (1) Take 5mL with a concentration of 1.0mol L -1 urea solution, 100mL amyl alcohol and 20mL water were mixed and stirred for 0.5h, 5mL methyl silicate was added to the mixture and stirred for 7h, the obtained suspension was centrifuged, washed with deionized water, and dried to obtain silica balls.
[0053] (2) 11.46g g urea and 1.7g Na 2 SnO 3 ·3H 2 O was dissolved in 200 mL of deionized water, then 18 mL of ethanol was added thereto, stirred, and a milky suspension was obtained. 3.82 g of the product of step (1) was ultrasonically dispersed in 4 mL of deionized water for 1 h at a molar ratio of Sn: Si: urea = 1:10:30 to obtain a suspension. The mixture obtained after mixing the two suspensions was transferred to a 300mL high-pressure furnace, heated at 2 atmospheres and 500°C for 12h, cooled, centrifuged, washed and dried. Take 240mg of the dried product, and mix it with 1.3ml, 2mol / L NaOH solution in molar ratio (Sn+Si):OH - =1:2, reacted for 12 hours, corroded ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com