Application of carbocyanine dye near infrared fluorescent compound
A carbocyanine dye and fluorescent compound technology, applied in the field of medicine, can solve the problems of enrichment, unclear photosensitive therapeutic effect of cyanine dyes, and lack of targeting properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-10
[0085] Particularly preferred carbocyanine compounds provided by the present invention
[0086]
[0087]
[0088]
Embodiment 11
[0090] In vivo tumor cell uptake and fluorescence imaging test
[0091] Materials and Method
Embodiment 1
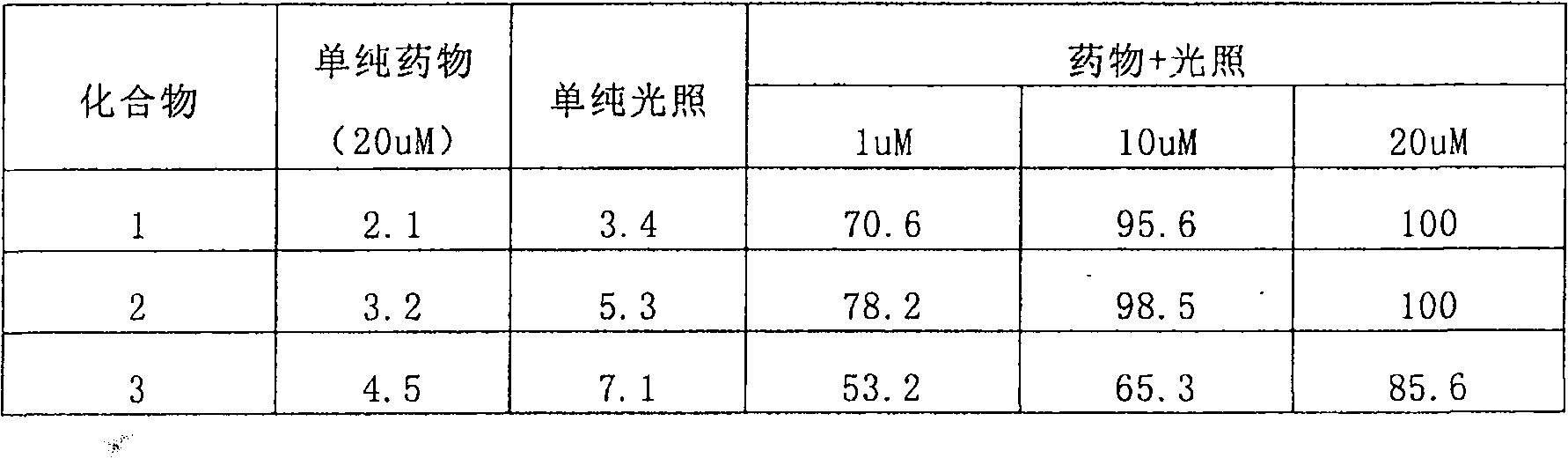
[0092] Preferred compounds 1, 2 and 3 provided in Examples 1, 2 and 3;
[0093] Human lung cancer H358 cells, purchased from ATCC, USA;
[0094] Kodak In-vivo Fx multifunctional live animal fluorescence imaging system, purchased from Kodak, USA.
[0095] Human lung cancer H358 cells were inoculated subcutaneously in nude mice to prepare a nude mouse tumor-bearing model. When the tumor grew to a size of 0.5-1 cm in diameter, the preferred compounds 1, 2 and 3 provided in Examples 1, 2 and 3 were administered through the tail vein 3. The dose is 0.2mg / kg body weight, and the fluorescence imaging of the tumor site is detected by Kodak In-vivo Fx multifunctional live animal fluorescence imaging system at different times after administration.
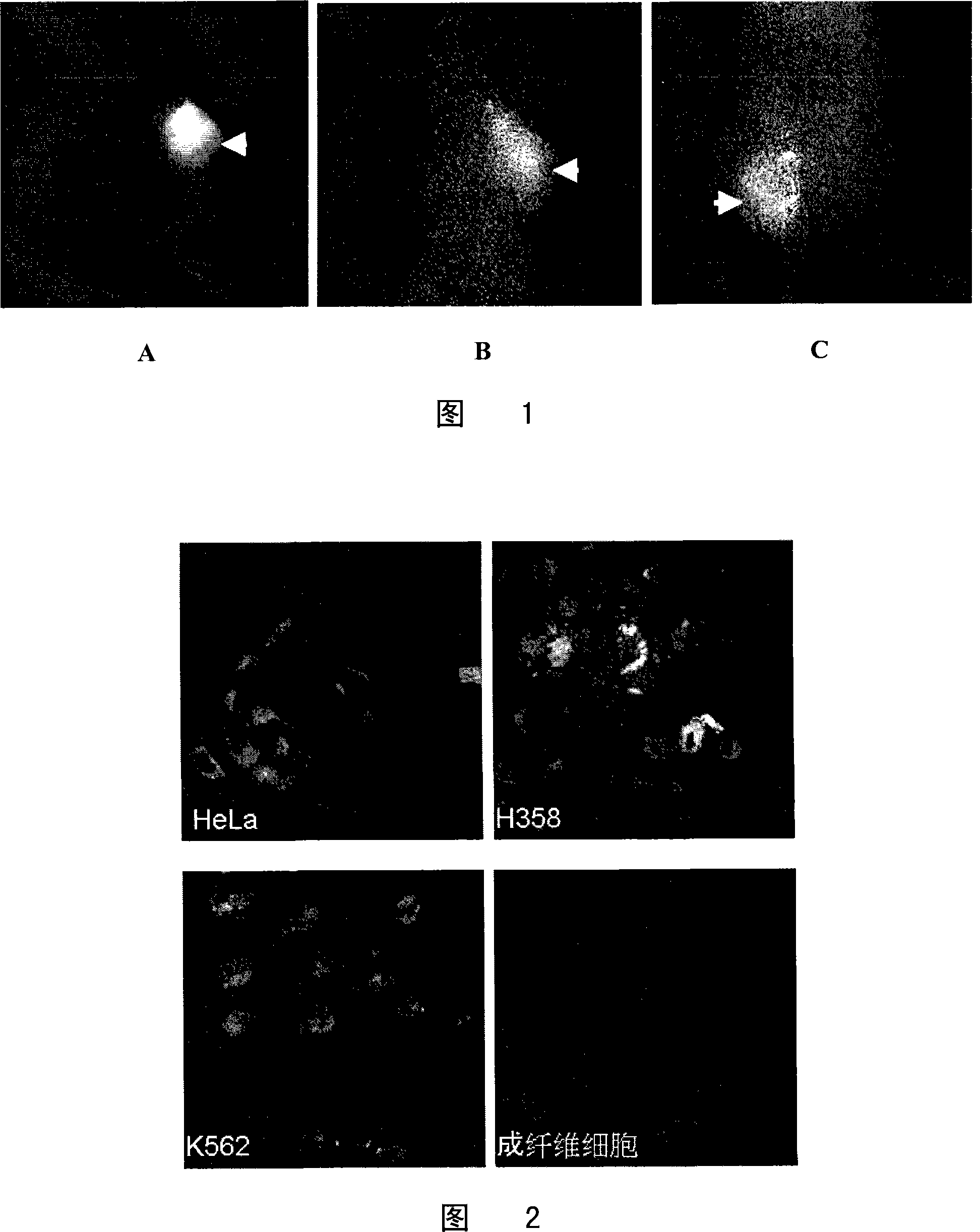
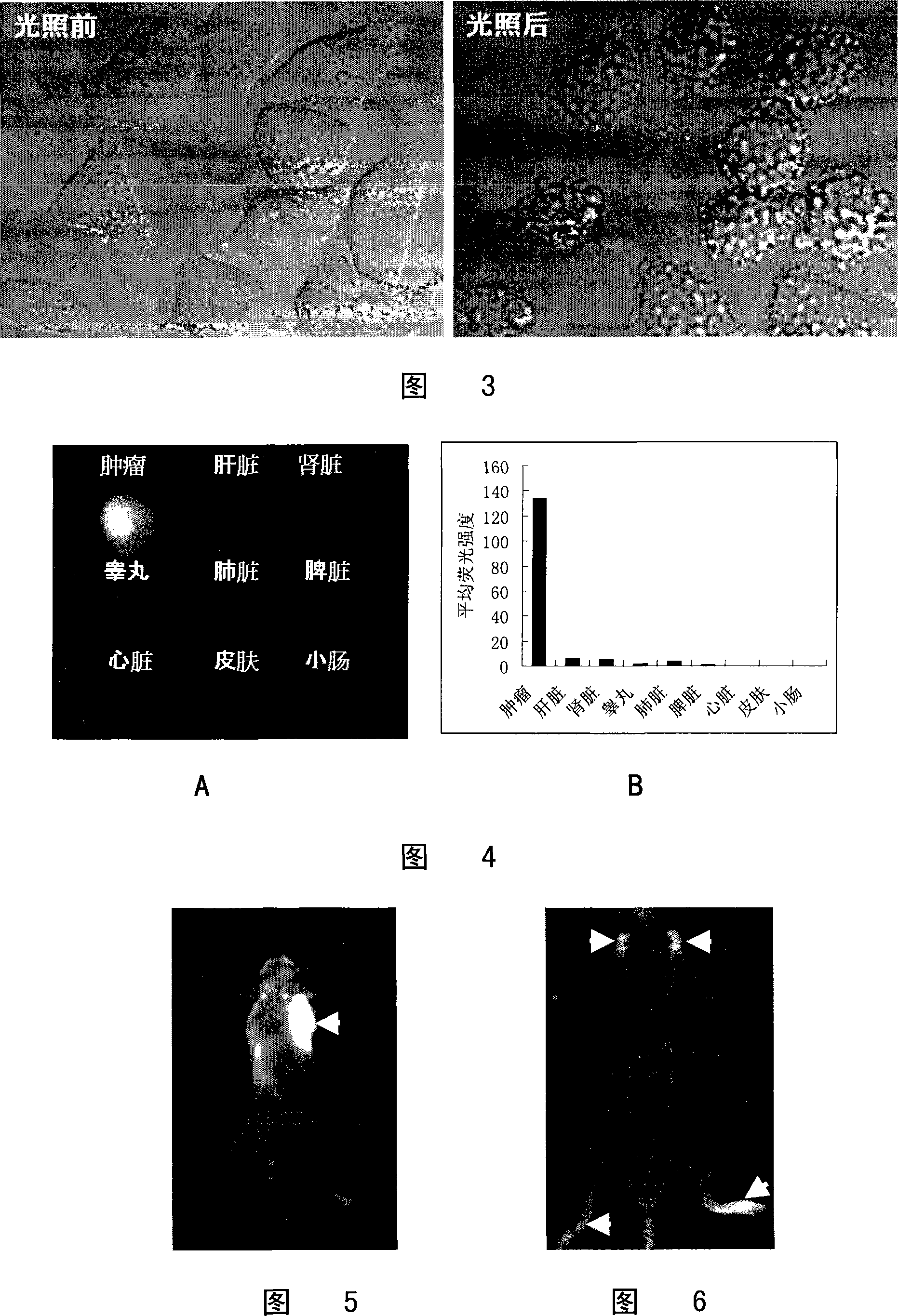
[0096] See the result figure 1 (Indicated by arrows): After the preferred compounds provided in Examples 1, 2 and 3 are administered to tumor-bearing animals, they are rapidly distributed throughout the body with blood circulation, and the overall f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com