Method for preparing oxide nano-materials by melted hydrated salt
A technology of nanomaterials and hydrated salts, applied in the field of functional oxide nanomaterials, can solve the problems of loss of water molecules, unseen nanomaterials, rising melting point, etc., and achieve the effects of avoiding pollution, reducing reaction temperature and high synthesis yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
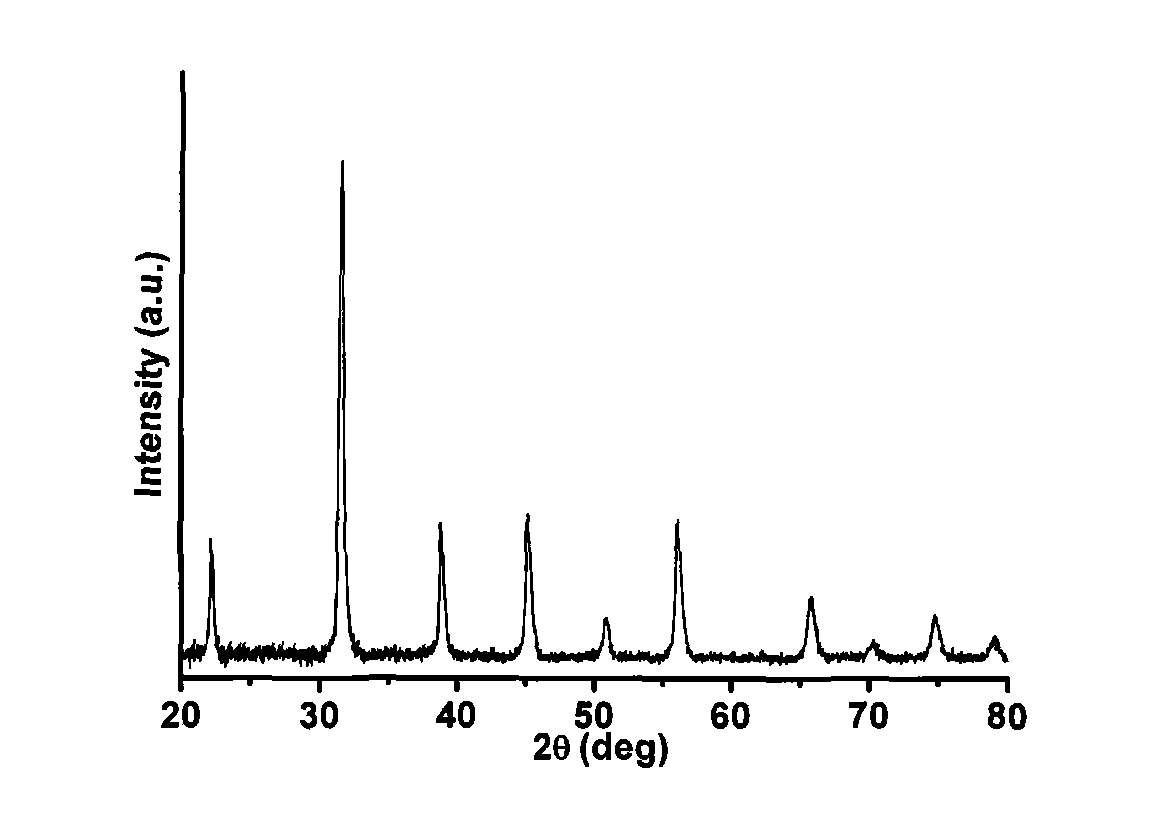
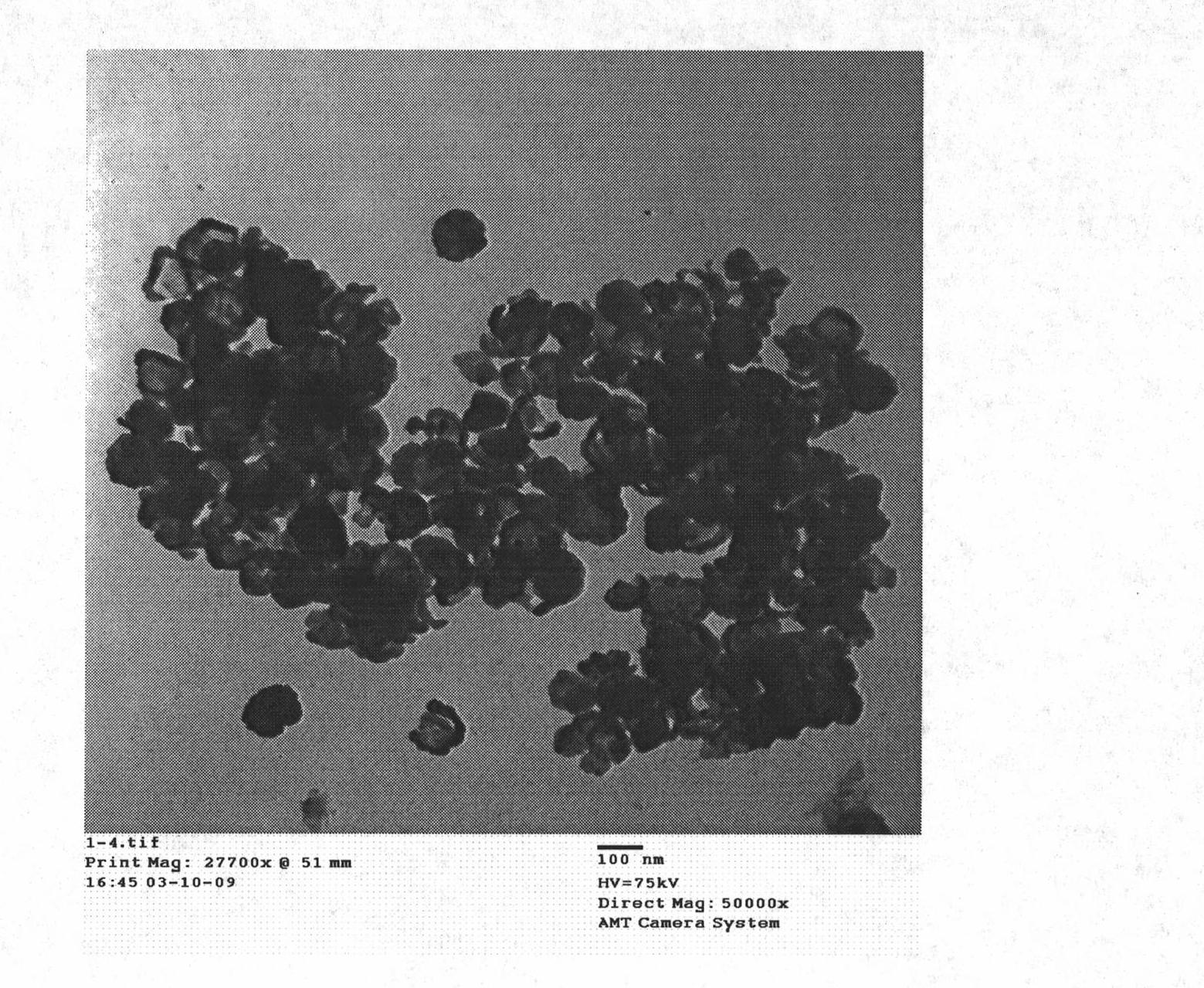
[0027] Embodiment 1: Preparation of BaTiO by molten hydrated salt method 3 hollow nanoparticles
[0028] 12g Ba(OH) 2 ·8H 2 O was placed in a 23mL airtight polytetrafluoroethylene container and heated at 90°C for 1 hour to melt;
[0029] Then open the airtight container and quickly add 0.8g TiO 2 Powder, stir evenly with a polytetrafluoroethylene rod, then reseal the container, put it in a constant temperature box, and react at a temperature of 180 ° C for 3 hours;
[0030]After cooling, take out the obtained solid product, dissolve and wash it with hot water at 50°C, and use a high-speed centrifuge to centrifuge the obtained mixture to separate the insoluble product;
[0031] The insoluble product was alternately washed 3 times with 95% ethanol and deionized water, and dried under normal pressure at 60°C for 1 hour to obtain monodisperse BaTiO 3 Hollow nanoparticles, weighing not less than 2.2g, the remaining liquid after the insoluble product is separated, and the hydra...
Embodiment 2
[0033] Embodiment 2: Preparation of BaTiO by molten hydrated salt method 3 nanoparticles
[0034] 8g Ba(OH) 2 ·8H 2 O was placed in a 23mL airtight polytetrafluoroethylene container and heated at 110°C for 40 minutes to melt;
[0035] Then open the airtight container and quickly add 0.6g homemade rutile TiO 2 powder (the rutile TiO 2 Utilize the pair containing 0.15M TiCl 3 NaCl saturated aqueous solution was subjected to hydrothermal treatment for 3 hours), stirred evenly with a polytetrafluoroethylene rod, then resealed the container, put it into a constant temperature box, and reacted at a temperature of 160 ° C for 1 hour;
[0036] After cooling, the obtained solid product was taken out, dissolved and washed with 0.1M dilute acetic acid solution, and the obtained mixed solution was separated by high-speed centrifugation to separate the insoluble product;
[0037] The insoluble product was alternately washed 4 times with 95% ethanol and deionized water, and dried natu...
Embodiment 3
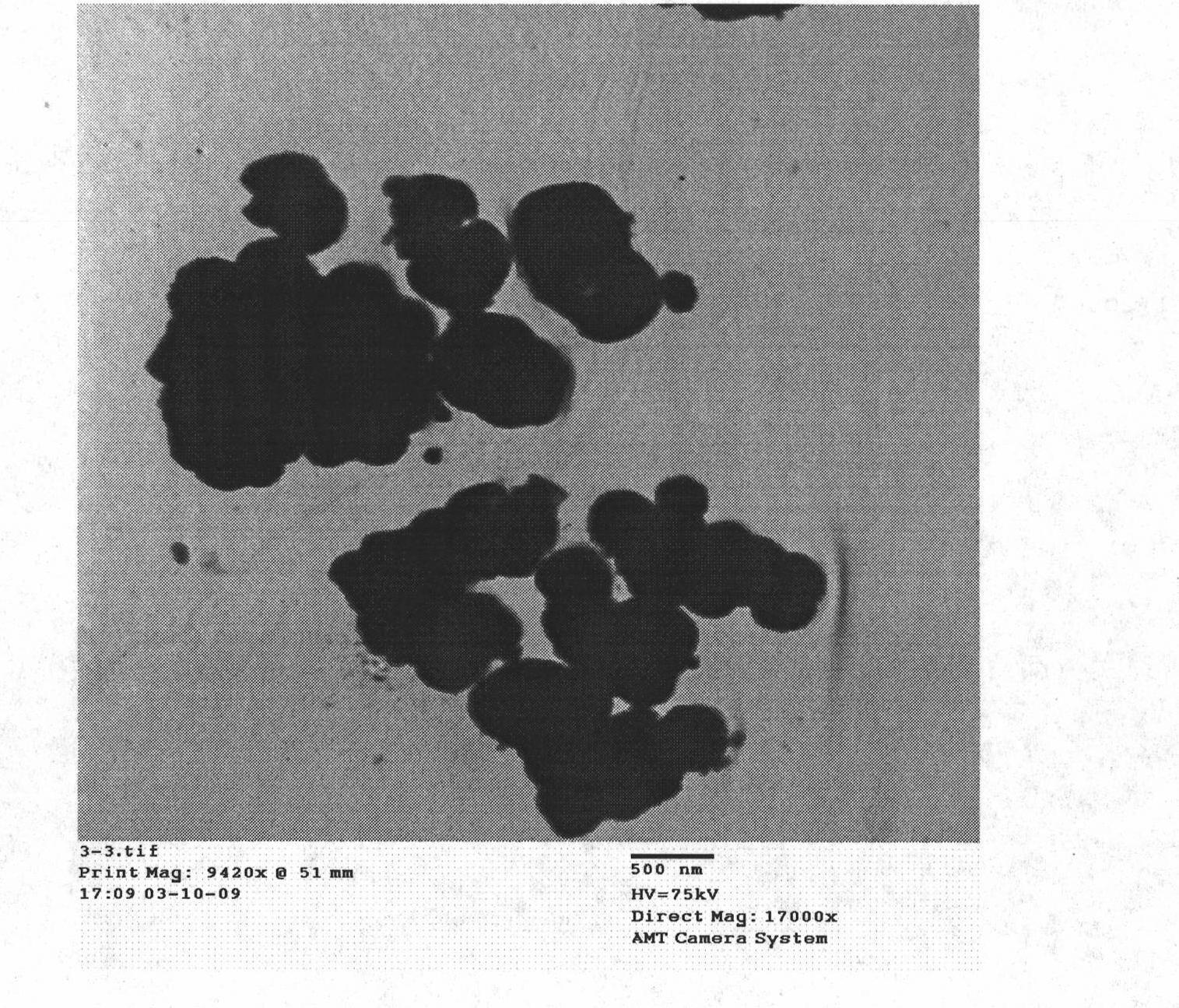
[0039] Embodiment 3: Preparation of SrTiO by molten hydrated salt method 3 nanoparticles
[0040] 12g SrCl 2 ·6H 2 O was placed in a 23mL airtight polytetrafluoroethylene container and heated at 130°C for 1 hour to melt;
[0041] Then open the airtight container and quickly add 1g TiO 2 , Stir evenly with a polytetrafluoroethylene rod, then re-seal the container, put it in an incubator, and react at a temperature of 170°C for 16 hours;
[0042] After cooling, take out the obtained solid product, dissolve and wash it with deionized water, and separate the insoluble product (particles larger than 200nm) from the obtained mixed solution with a vacuum pumping rate;
[0043] The insoluble product was alternately washed 3 times with 95% ethanol and deionized water, and dried in vacuum to obtain SrTiO 3 Nanoparticles, weighing no less than 2.1g, with a size of about 150-300 nanometers, the remaining liquid after the insoluble product is separated, and recrystallized to obtain a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com