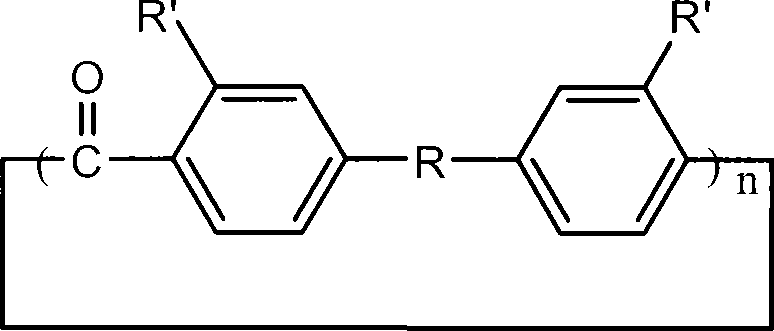
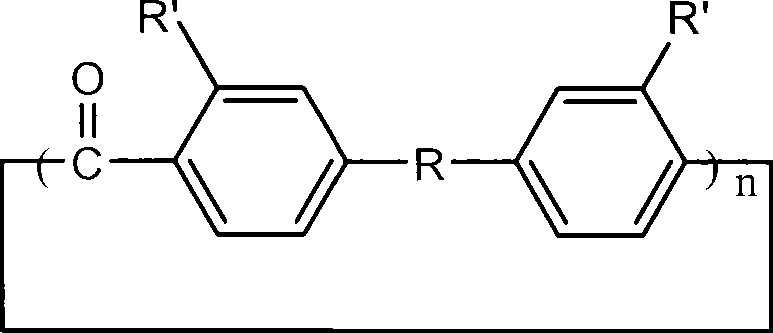
Aromatic ring-shaped polyether-ketone oligomer and preparation method thereof
An oligomer and polyether ketone technology, which is applied in the field of high-performance polymer precursors, can solve problems such as polymer performance degradation, and achieve the effects of low cost, high yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Preparation of Polyetherketone Cyclic Oligomer
[0024] Put 6.68g (0.05mol) of anhydrous aluminum trichloride and 500mL of carbon disulfide into a 1L three-neck flask with a spherical condenser connected to a calcium chloride drying tube and a gas absorption device at the top. Dissolve 3.0812g (0.02mol) of carbon tetrachloride and 7.3258g (0.02mol) of 4,4'-phenoxybenzophenone in 100mL of carbon disulfide, and drop the solution slowly and uniformly within 8 hours under room temperature and vigorous stirring Add it into a three-necked flask, after the dropwise addition, continue to stir for 2 hours; add 80 mL of 0.1M dilute hydrochloric acid to terminate the reaction, and continue to stir for 2 hours. After the organic phase was obtained by liquid separation, it was washed three times with 100 mL of distilled water to remove the catalyst. Concentrate and add dropwise to methanol to precipitate to obtain a cyclic oligomer as a light yellow powder with a yield of...
Embodiment 2
[0025] Example 2 Preparation of Polyetheretherketone Cyclic Oligomer
[0026] The reaction monomer 5.2442g (0.02mol) triphenyl ether was replaced by 4,4'-phenoxybenzophenone, and other monomer feed ratios, reaction conditions and processing steps were the same as in Example 1. The product is a light yellow powder with a yield of 74%. The main signal peaks of the laser mass spectrogram of the product are located at: m / z=577.4, 865.5, 1153.5, 1441.4, 1729.9, 2018.1, corresponding to the protonated molecular ions of the dimer to the heptamer of the cyclic oligomer peaks, indicating that the product is composed of a series of cyclic oligomers with different degrees of polymerization.
Embodiment 3
[0027] Example 3 Preparation of Polyetheretherketone Cyclic Oligomer
[0028] Replace 4,4'-phenoxybenzophenone with 5.2442g (0.02mol) triphenyl ether as the reaction monomer, replace carbon disulfide with nitromethane as the solvent, and other monomer feed ratios, reaction conditions and processing steps are the same as in Example 1 . The product was a light yellow solid with a yield of 78%. The main signal peaks of the laser mass spectrogram of the product are located at: m / z=577.6, 865.4, 1154.0, 1441.7, 1729.8, 2018.0, 2306.1, corresponding to the protonation of the dimer to the octamer of the cyclic oligomer Molecular ion peaks, indicating that the product is composed of a series of cyclic oligomers with different degrees of polymerization.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com