Method for preparing andrographolide derivatives and preparation thereof
A technology of andrographolide and derivatives is applied in the field of medicine and achieves the effects of stable drug quality and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
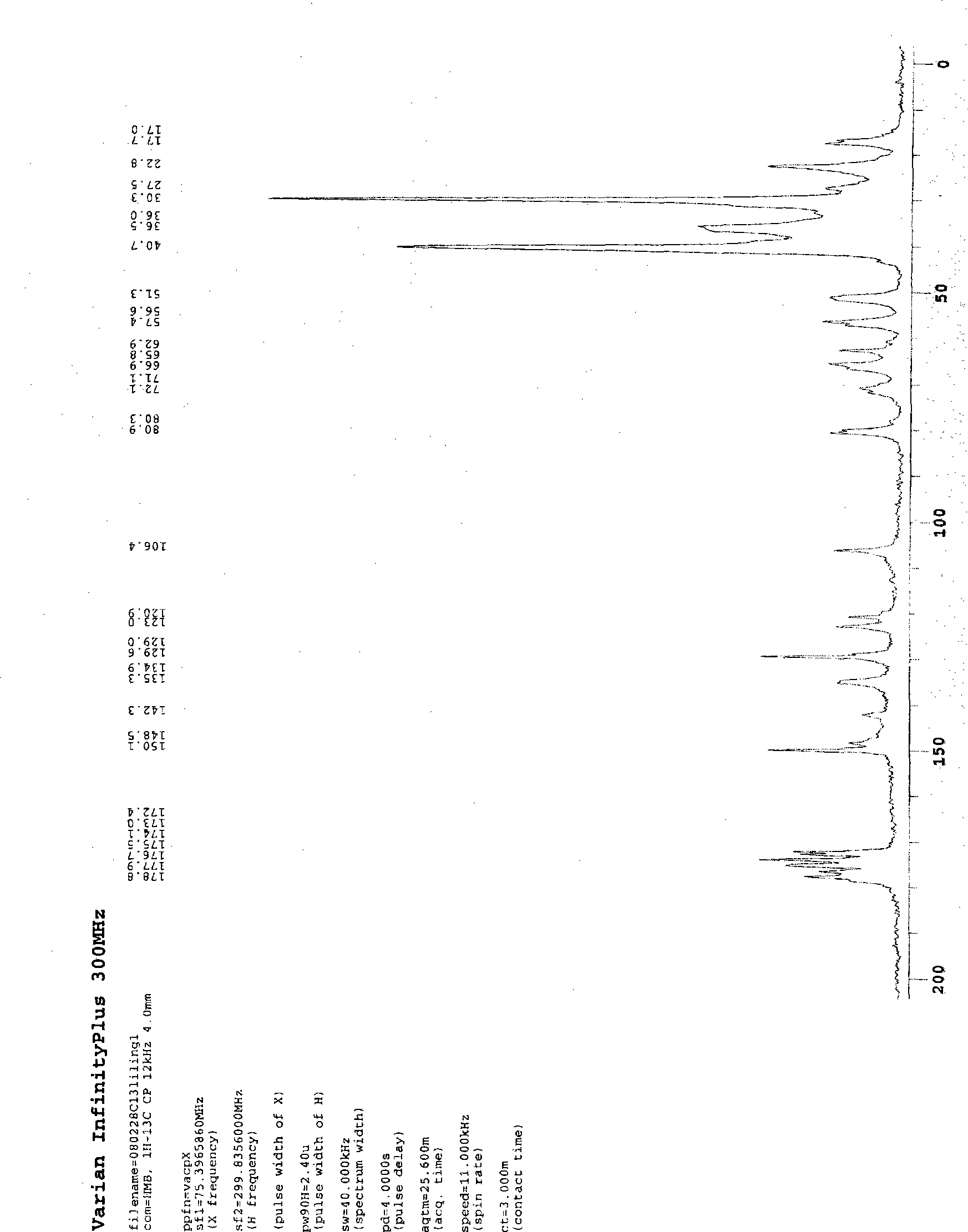
[0016]
[0017] 30.3 (N-C-CH 2 - C h 2 ).
[0018] MW 187.7 C 10 h 17 N HCl
[0019] Physical and chemical properties: slightly soluble in water, soluble in chloroform. MP180~192℃. Hydrochloride was dissolved in 5 times ethanol, 18 times chloroform, 2.5 times water. Ph3.5-5, 1.87g amantadine hydrochloride (0.01mol) + 5ml distilled water dissolved, 25% NaOH (1.6ml, theoretical amount) to adjust Ph9-10, a large amount of solid precipitated, washed with water and dried. MP215~225℃
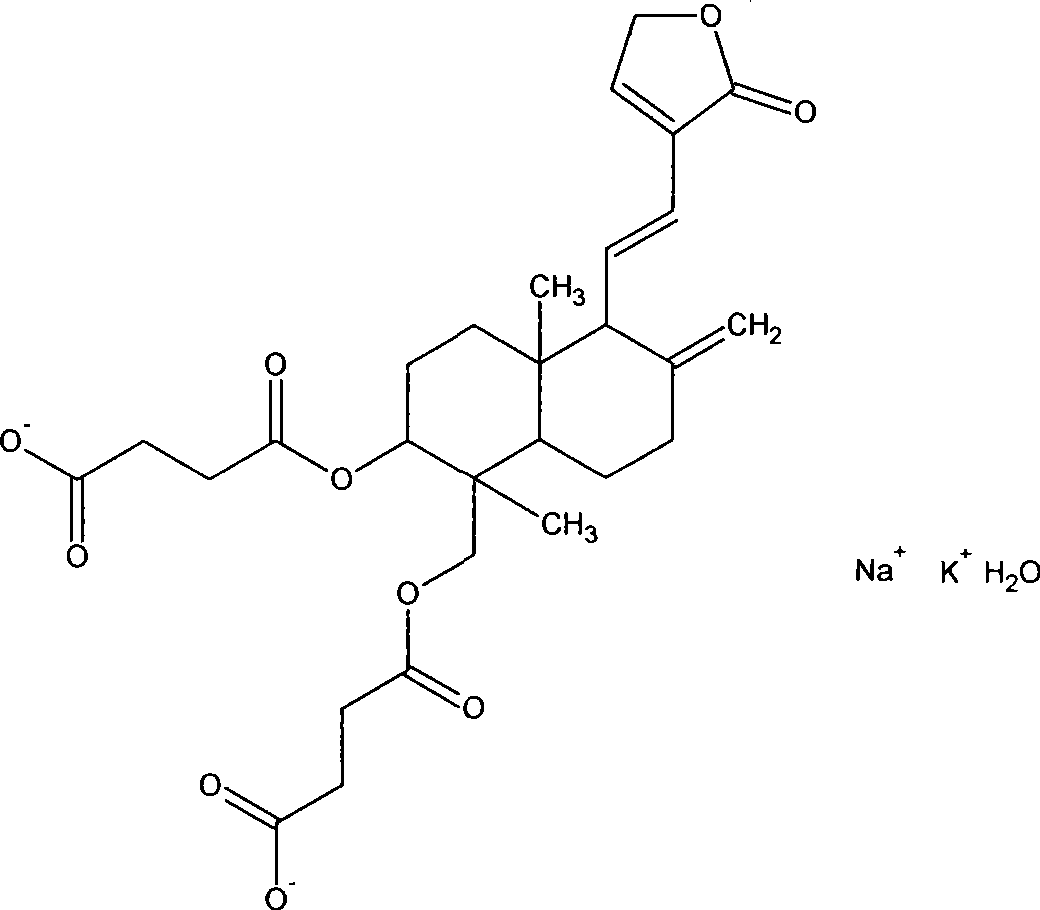
[0020] Yanhuning
[0021]
[0022] MW C 28 h 34 KNaO 10 h 2 O 610.6 Ph6.0-8.0 unstable.
[0023]
[0024] Andrographolide Disuccinate Half Ester MW C 28 h 36 o 10 532.5 MP Measured at 102-110°C (137-140°C literature) andrographolide disuccinic acid half ester 2.67g (0.005mol) was added to 40ml of chloroform, slightly heated to dissolve. Add 1.51 g (0.01 mol) of amantadine into 30 ml of chloroform to dissolve. Under stirring at room temperature, the amantadine / chloroform so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com