Static spraying composite film and preparation method and application thereof
A composite film and electrostatic spraying technology, applied in the field of materials, can solve the problems of waste of cotton yarn resources, easy drying of dressings, pain of patients, etc., and achieve the effects of promoting wound healing, increasing flexibility, and wide-ranging antibacterial properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
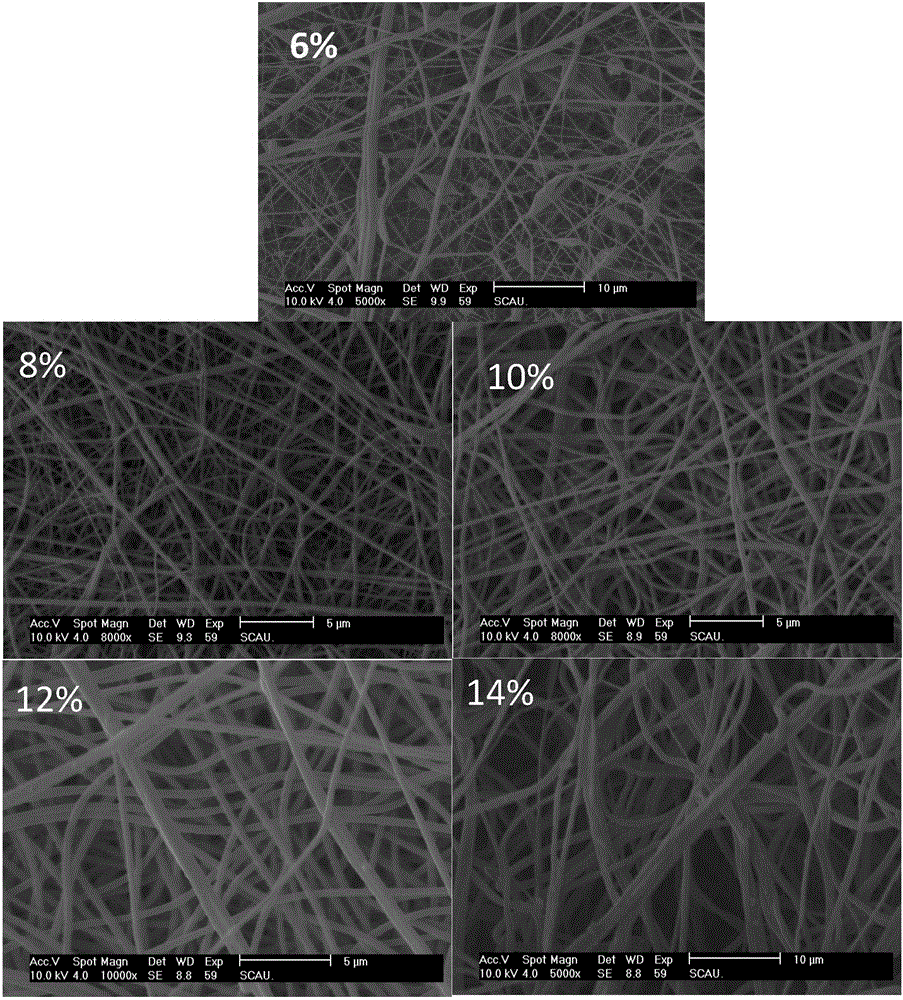
[0071] (1) Dissolve PVDF powder in acetone / dimethylacetamide DMAC (the ratio of acetone and DMAC is 1:1 by mass) mixed solvent, and prepare the mass fractions of 6%, 8%, 10%, and 12% respectively , 14% spinning solution. At room temperature, the solution was airtightly stirred on a magnetic stirrer for 6 hours, ultrasonic (power 120W, frequency 40,000Hz,) 2 hours, to ensure that the polymer was completely dissolved, and a homogeneous solution was obtained. Spinning: 10ml PVDF spinning liquid is spun at 15KV, receiving distance 15cm, using aluminum foil as receiving medium, and the flow rate is 0.5mL / h;
[0072] (2) The electrospun membrane was vacuum filtered and dried (drying pressure 10mpa, drying temperature 45°C, drying time 24h), to remove residual organic solvents, vacuum filtered and dried, and then analyzed by scanning electron microscope, the results were as follows figure 1 shown.
[0073] figure 1 SEM images and diameter distribution images of nanofibers prepared...
Embodiment 2
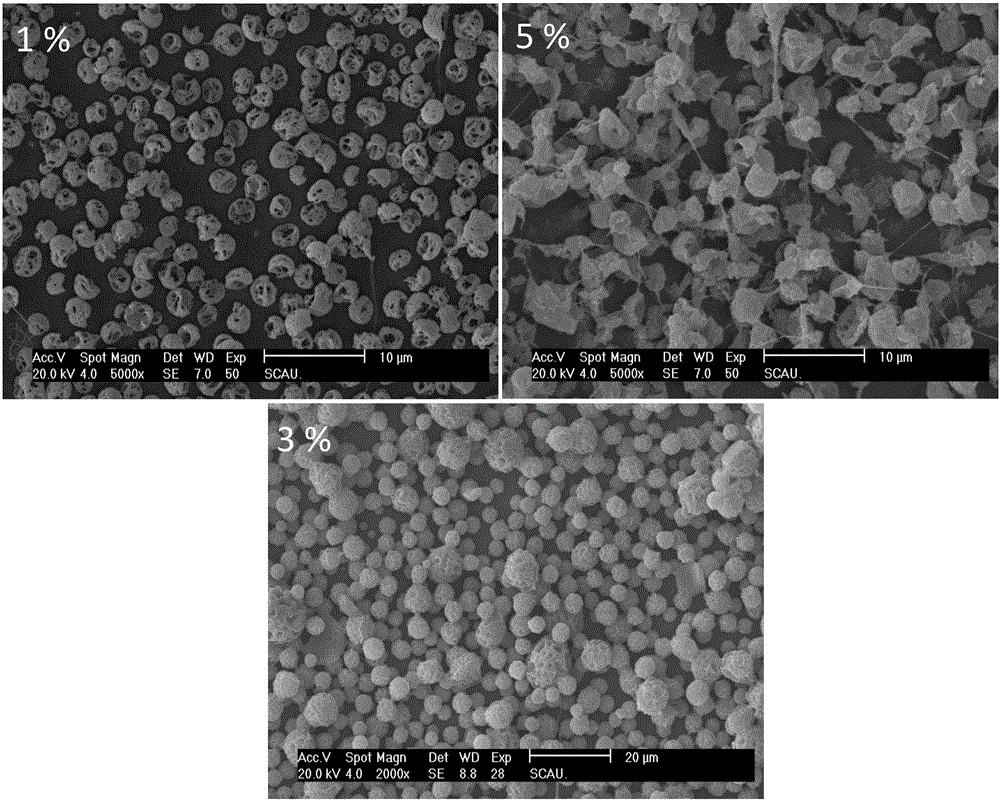
[0075] (1) PLA particles were dissolved in chloroform to form spinning solutions with a mass fraction of 1%, 3%, and 5%, respectively, and Cur was added to the PLA spinning solution to prepare a mass ratio of PLA to Cur of 85 / 15. At room temperature, the solution was airtightly stirred on a magnetic stirrer for 6 hours, and ultrasonic (power 120W, frequency 40,000Hz) was used for 2 hours to ensure that the PLA polymer and drug were completely dissolved to obtain a transparent solution. for subsequent spinning. 10ml of Cur-PLA spinning solution was prepared under the conditions of 17KV, receiving distance of 15cm, and flow rate of 0.5mL / h.
[0076] (2) The electrospun membrane was vacuum filtered and dried (drying pressure 10mpa, drying temperature 45°C, drying time 24h), to remove residual organic solvents, vacuum filtered and dried, and then analyzed by scanning electron microscope, the results were as follows figure 2 shown.
[0077] figure 2 For the same solvent (chlor...
Embodiment 3
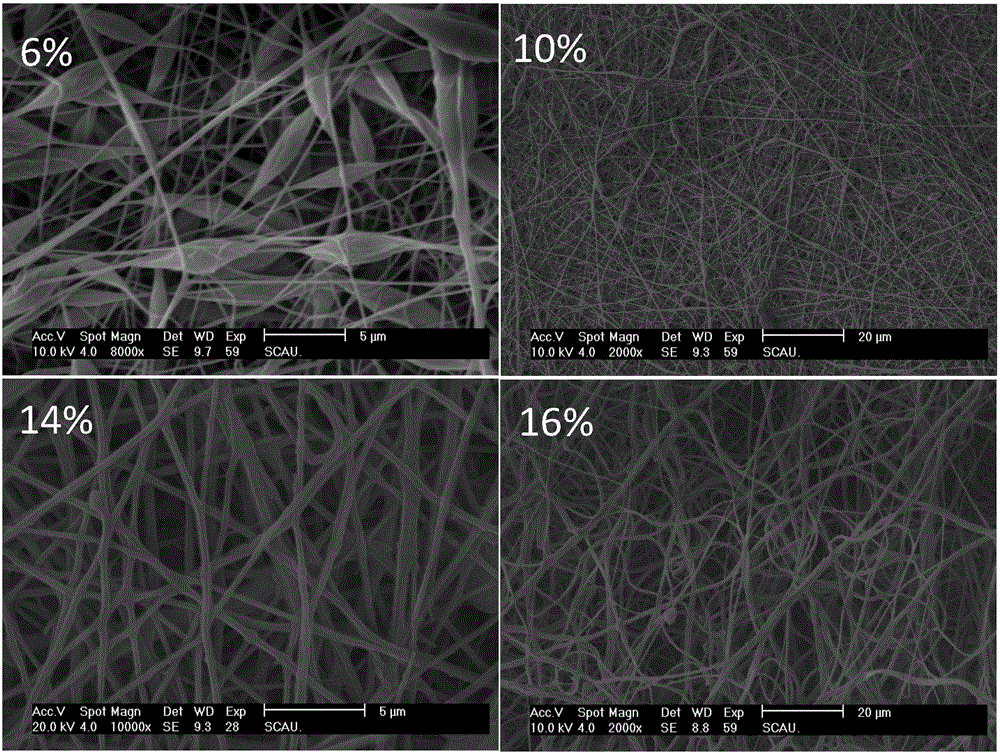
[0079] PLA particles were dissolved in acetone / DMAC (1:1, m / m) mixed solvent to prepare electrospinning solutions with mass fractions of 6%, 10%, 14%, and 16%, respectively. Add enrofloxacin solid drug into the PLA spinning solution to prepare the mass ratio of Enro to PLA as 20 / 80. At room temperature, the solution was airtightly stirred on a magnetic stirrer for 6 hours, and ultrasonic (power 120W, frequency 40,000Hz) was used for 2 hours to ensure that the PLA polymer and drug were completely dissolved to obtain a transparent solution. for subsequent spinning. 10ml of spinning dope was prepared under the conditions of a unified electrospinning process with a spinning voltage of 15KV, a receiving distance of 10cm, and a flow rate of 0.5mL / h.
[0080] The electrospun membrane was vacuum-filtered and dried (drying pressure 10mpa, drying temperature 45°C, drying time 24h) to remove residual organic solvents. After vacuum-filtering and drying, the morphology was analyzed with a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com