Andrographolide derivative, salt, preparation method and application thereof
A technology of andrographolide and its derivatives, applied in chemical instruments and methods, drug combinations, pharmaceutical formulations, etc., can solve problems such as toxic side effects and quality control difficulties, and achieve good biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] This example discloses the preparation process of 19-O-phosphate monosodium salt-14-O-methylandrographolide
[0034] 1.1. Synthetic route design
[0035]
[0036] 1.2.3, Preparation of 19-O-isopropylidene andrographolide (CXL-1)
[0037]
[0038] Andrographolide (5.0 g, 14.3 mmol) was dissolved in dry DMF (100 mL), DMP (20 mL, 0.16 mol) and p-TsOH (272.0 mg, 1.4 mmol) were added under argon protection, and the reaction was stirred overnight at room temperature. After the reaction was detected by TLC, triethylamine (1 mL) was added to terminate the reaction. The reaction solution was diluted with EtOAc (200ml), washed with saturated NaHCO 3 (3×100mL), washed with saturated brine (2×100mL). The organic phase was dried over anhydrous sodium sulfate, filtered, and concentrated by silica gel column chromatography (3:1-1:1, petroleum ether-EtOAc) to give white solid CXL-1 (4.54 g, 81.5%). R f 0.53 (1:1, Petroleum ether-EtOAc,).
[0039] 1 H NMR (500MHz, DMSO): δ...
Embodiment 2
[0058] Weigh 1 g of CXL-5, dissolve it in MeOH (20 mL), add 1M KOH in MeOH dropwise under ice-cooling, adjust the pH to 8.5-9.0, and naturally rise to room temperature and stir overnight. The methanol solution of 1M HCl was added dropwise to pH = 7.0 to terminate the reaction, the solvent was distilled off under reduced pressure, and the 2 O / isopropanol, H 2 O / acetone recrystallized several times to obtain 14-O-methylandrographolide-19-O-phosphate monopotassium salt (530.7 mg).
Embodiment 3
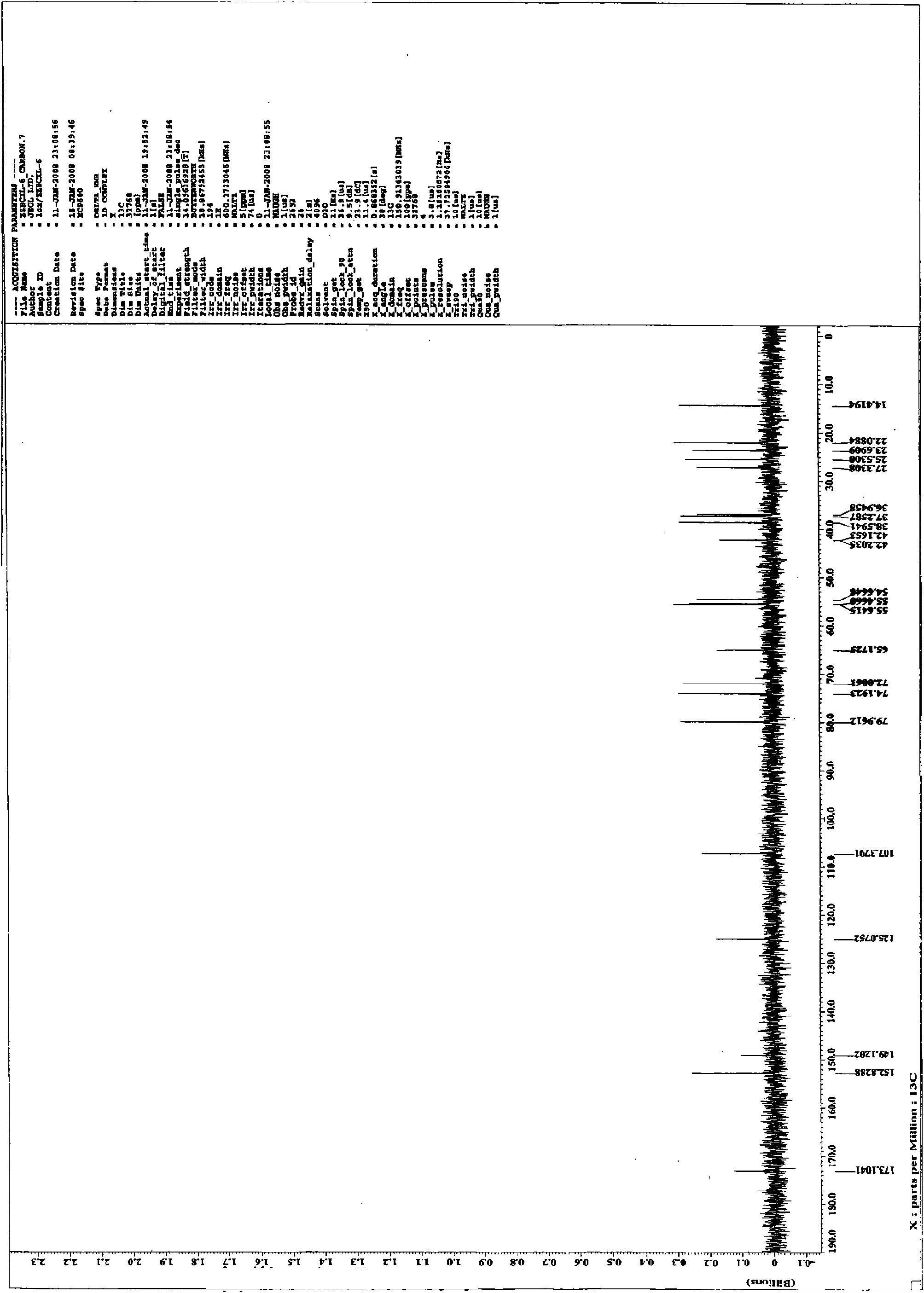
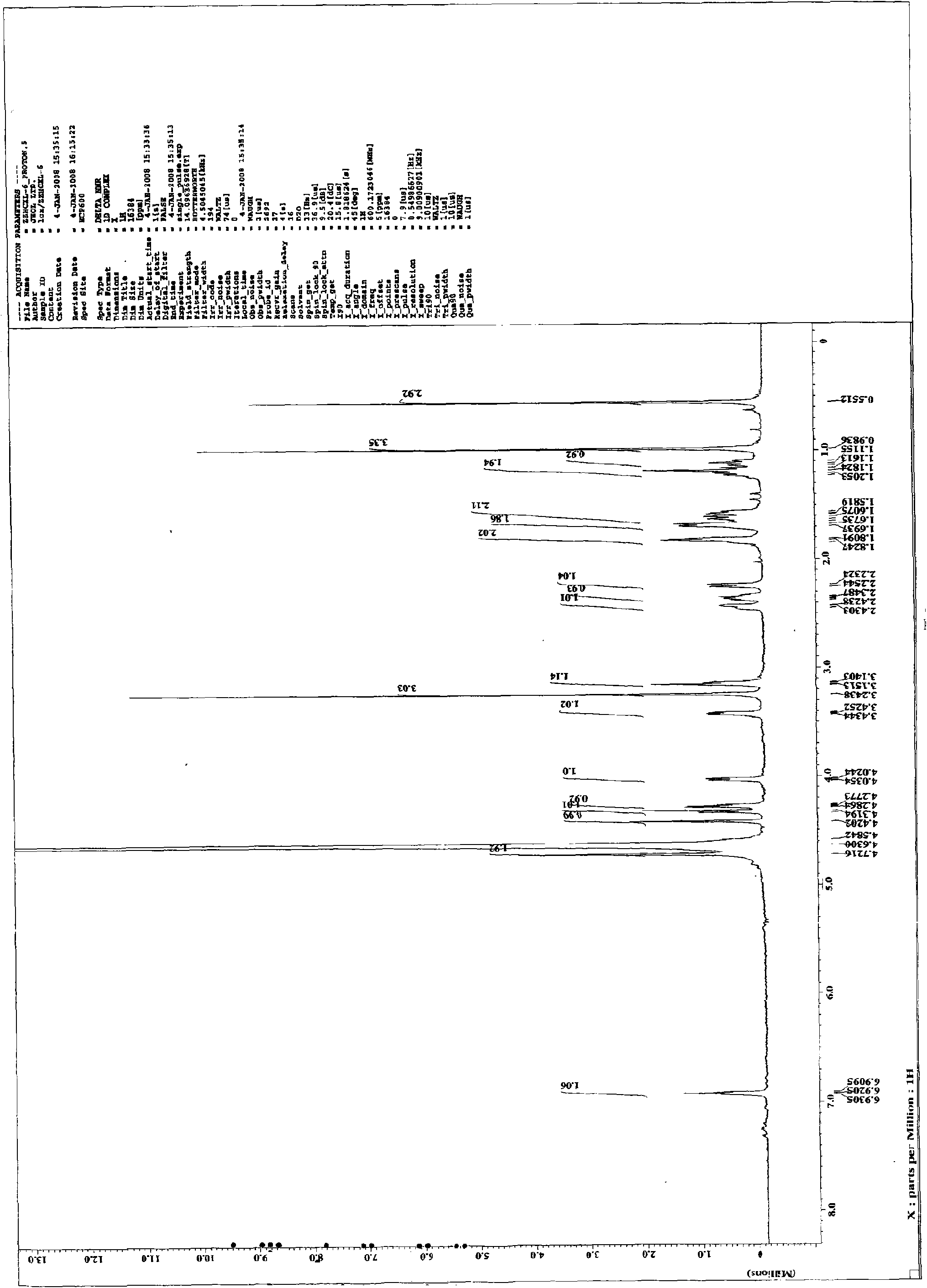
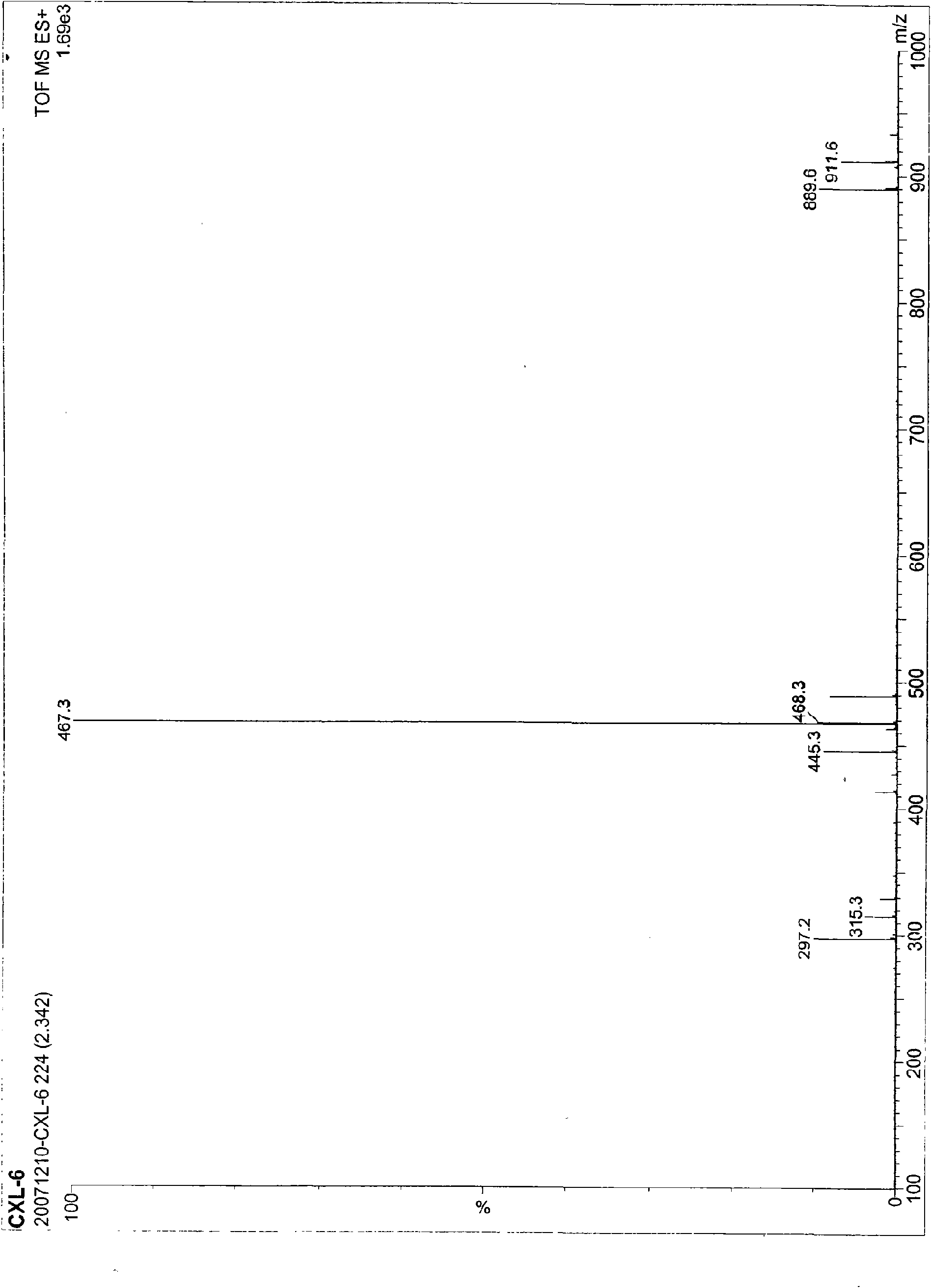
[0060] This example discloses the inhibitory effect of 14-O-methylandrographolide-19-O-phosphate monosodium salt (CXL-6) on the secretion of NO from macrophages induced by LPS:
[0061] 1. Drugs and instruments:
[0062] Lipopolysaccharide (LPS) and MTT were purchased from Sigma; mouse macrophage RAW 264.7 was purchased from the Cell Bank of the Chinese Academy of Sciences (ATCC); RPMI 1640 medium, penicillin, streptomycin, and fetal bovine serum were purchased from Gibco; others Commonly used biochemical reagents are all domestically produced analytically pure; automatic microplate reader (produced by Biotek Group in the United States); constant temperature CO 2 Incubator (the United States, produced by Thermo Company). Andrographolide was purchased from the market and dissolved in DMSO for in vitro cell experiments. 14-O-methylandrographolide-19-O-phosphate monosodium salt was prepared according to the method in Example 1, and Chuanhuning, Lianbizhi and Xiyanping were purc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com