Kit and method for modifying vitro synthesized RNA
An in vitro synthesis and kit technology, applied in the field of nucleic acid research, to achieve mild conditions, high yield and good modification effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1: Construction of a kit for modifying RNA synthesized in vitro
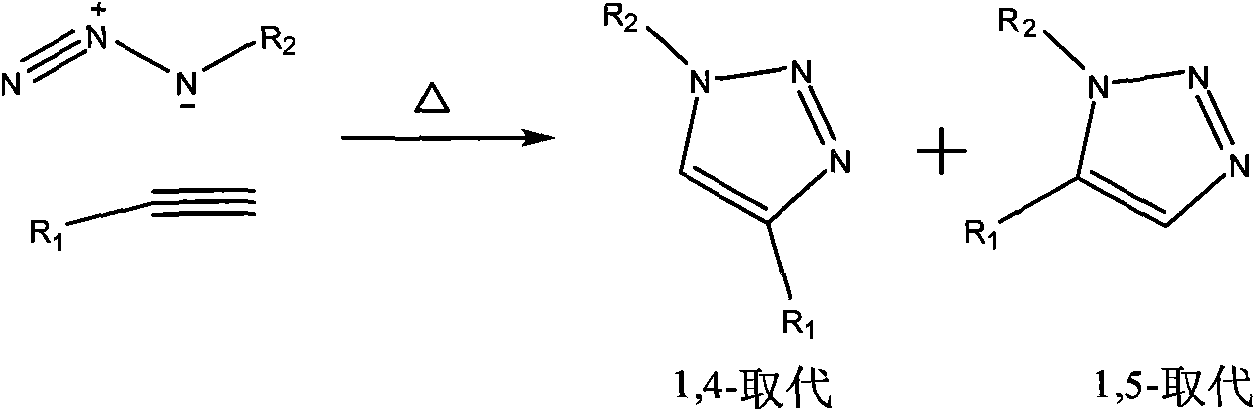
[0059] (1) Synthesis of phosphoramidite monomers carrying 1,3-dipolar groups and dipolar-philic groups
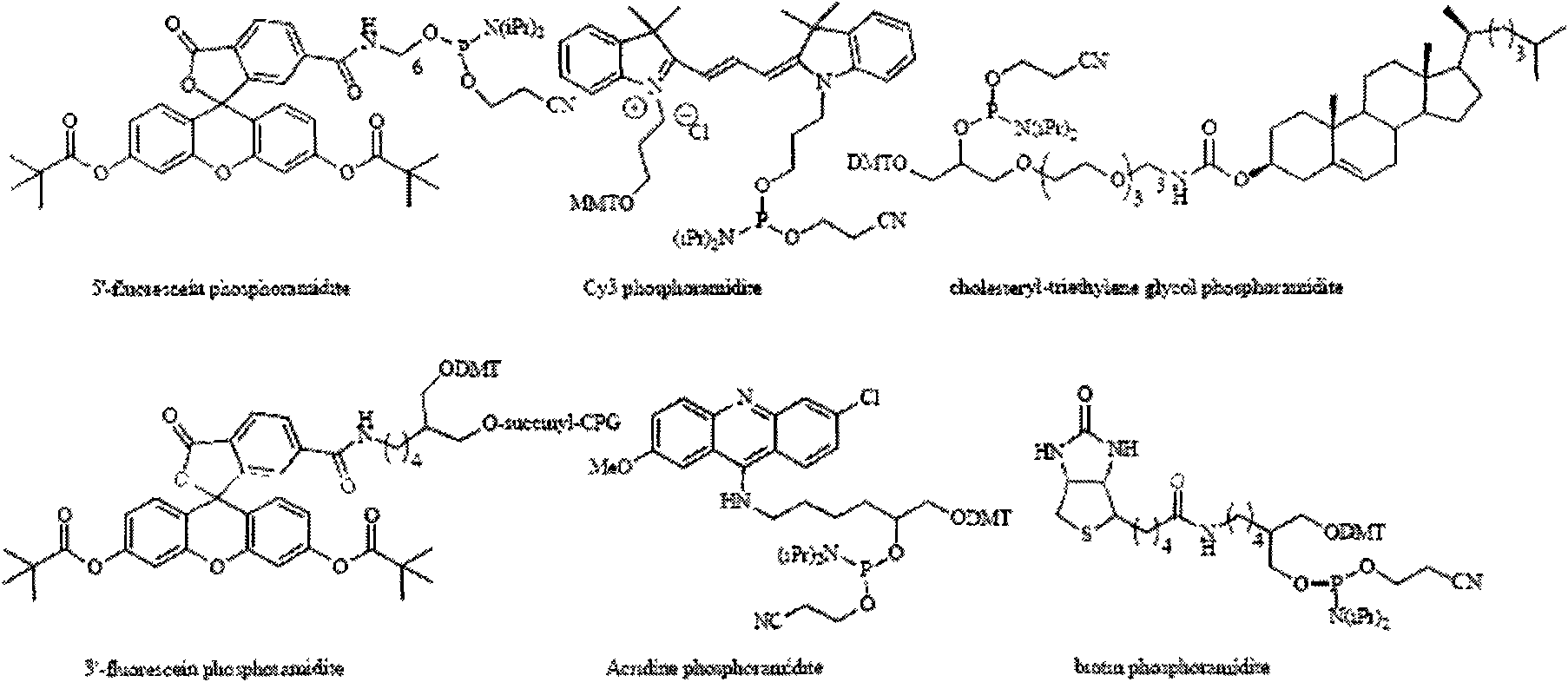
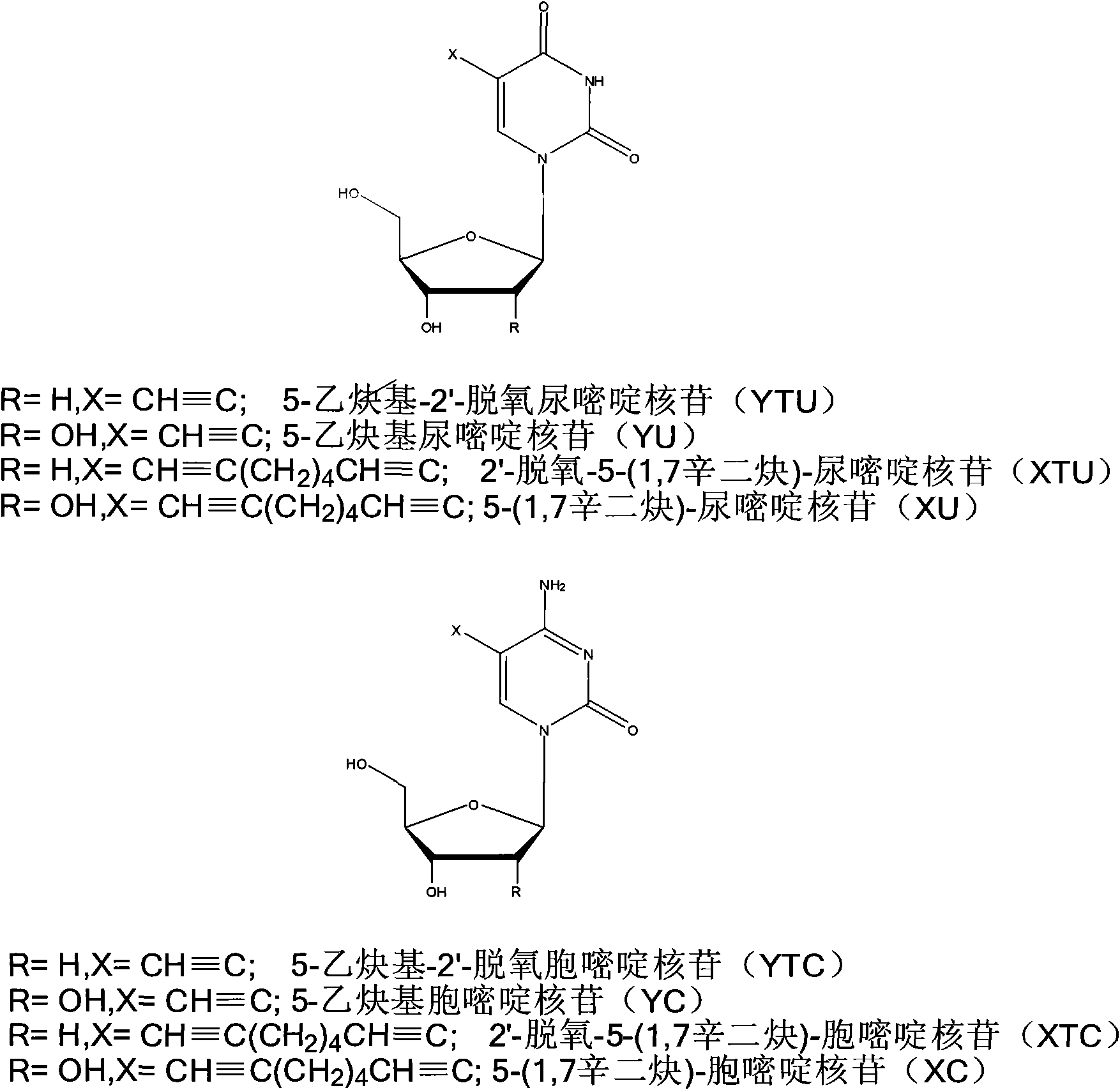
[0060] The present invention has synthesized a series of phosphoramidite monomers carrying 1,3-dipolar groups and dipolar-philic groups, including alkynyl glycerol phosphoramidites, alkynyl triethylene glycol phosphoramidites and Figure (3)-( 6) The phosphoramidite monomer corresponding to the nucleoside. These monomers are synthesized by reacting corresponding substrates with 2-cyanoethyl N, N-diisopropyl phosphoramidite chloride, so only alkynyl glycerol phosphoramidite and alkynyl triethylene glycol phosphoramidite , 5-ethynyl uridine phosphoramidite synthesis as an example.
[0061] Synthesis of Alkyglycerol Phosphoramidites (2)
[0062]
[0063] 0.5g (2.66mmol) of alkynyl triethylene glycol and 0.75ml (5.31mmol) of diisopropylamine were dissolved in 2ml of anhydrous dichloromethane, ...
Embodiment 2
[0111] Example 2: RNA is modified during different stages of synthesis
[0112] Use one or more of the following methods to obtain functional molecularly modified RNA.
[0113] "Click" reaction during abasic protection in RNA synthesis:
[0114] RNA carrying a dipolar group: In chemical solid-phase synthesis, alkynyl phosphoramidites (such as alkynyl glycerol phosphoramidites, alkynyl triethylene glycol phosphoramidites) are used as substrates to modify and synthesize RNA to introduce alkynyl groups;
[0115] Biomolecular probes carrying 1,3-dipolar groups: 1,1,1',1'-tetramethyl-3-ethyl-3'-azidopropyl-benzindole trimethine cyanine;
[0116] Catalyst: Cu(CH 3 EN) 4 PF 6 ;
[0117] Solvent: ethanol ammonia solution;
[0118] 100nmol of unprotected dipolar group-carrying RNA was dissolved in ethanol ammonia solution, 200nmol of 1,3-dipolar group-carrying biomolecular probe was added, and 100nmol of catalyst was heated overnight. After the ammonia water was spin-dried, it ...
Embodiment 3
[0152] Example 3: Cholesterol linked to RNA
[0153] RNA carrying a dipolar group: In chemical solid-phase synthesis, the RNA is modified with an alkynyl phosphoramidite to introduce an alkynyl group;
[0154] Biomolecular probes carrying 1,3-dipolar groups: azidocholesterol;
[0155] Catalyst: C 54 h 45 BrP 4 Cu;
[0156] Solvent: tetrahydrofuran.
[0157] 100nmol of undeprotected 2'-position RNA carrying dipolar group was dissolved in 100μl tetrahydrofuran, and 100nmol C was added 54 h 45 BrP 4 Cu, 500nmol azidocholesterol, and 1000nmol tetrabutylammonium fluoride were reacted for 4-12 hours, and the reaction solution was evaporated to dryness, followed by standard RNA post-processing methods to finally obtain 76nmol RNA linked to cholesterol molecules.
[0158] The connection method of other long-chain alkanes and non-polar active molecules (such as: liposome molecules, hydrophobic drugs, etc.) is the same as that of cholesterol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com