Novel compounds and methods for their production
A compound, representative technology, applied in the field of producing these compounds, can solve the problem of reducing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
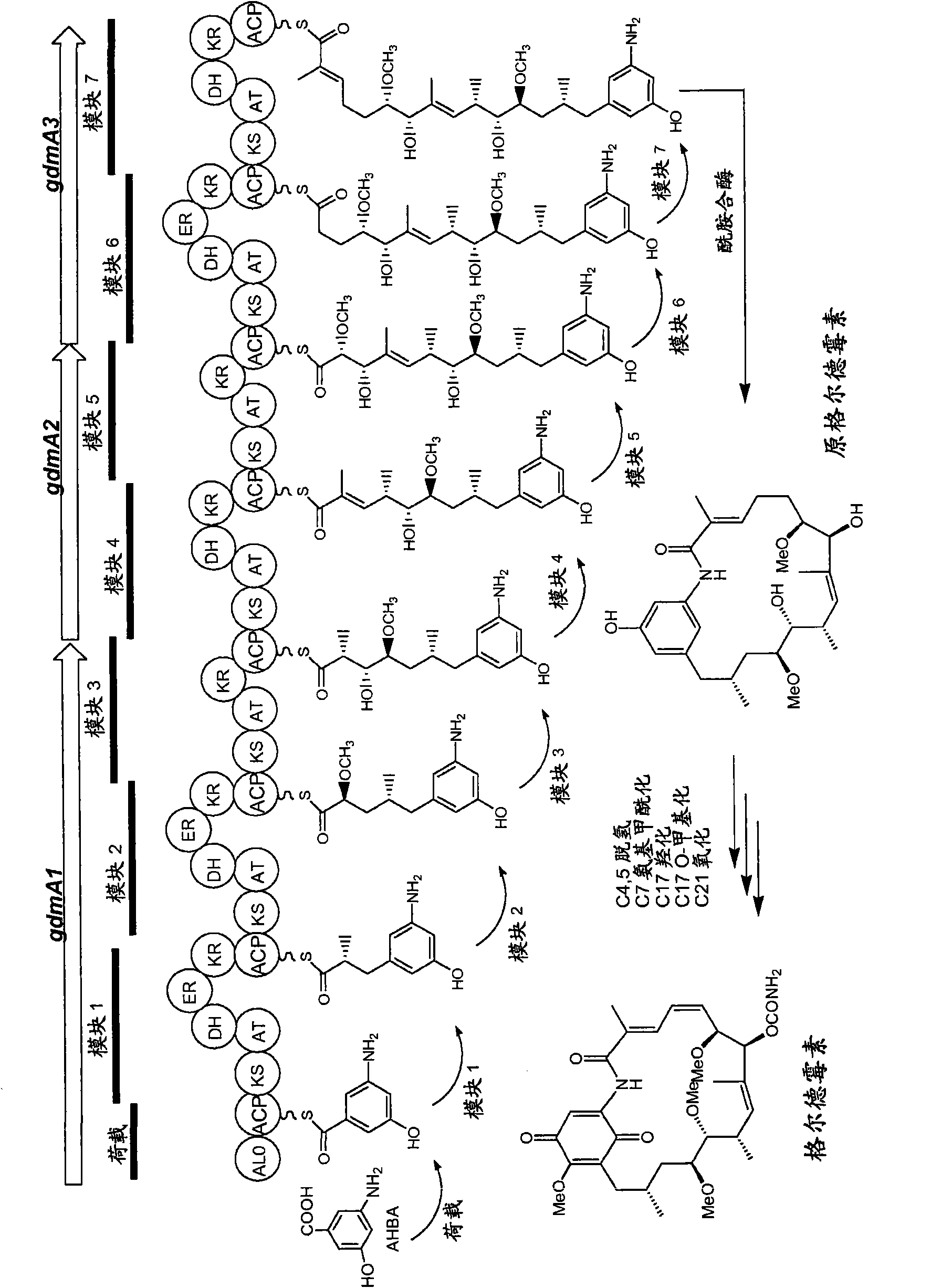
[0345] Example 1 - Sequencing of the Osbaycin PKS Gene Cluster
[0346] Genomic DNA was isolated from A. preliminaris (ATCC 31280) and A. miraculouse (DSM 43827, ATCC 29888) using standard protocols described by Kieser et al. (2000). DNA sequencing was performed using standard methods by the Sequencing Laboratory at the Biochemistry Department, University of Cambridge, Tennis Court Road, Cambridge CB2 1QW, Tennis Court Road, Cambridge CB2 1QW.
[0347] Using primers BIOSG104 5'-GGTCTAGAGGTCAGTGCCCCCGC GTACCGTCGT-3' (SEQ ID NO: 1) and BIOSG105 5'-GGCATATGC TTGTGCTC GGGCTCAAC-3' (SEQ ID NO: 2) amplified from Streptomyces hygroscopicus NRRL 3602 Gell The carbamoyltransferase encoding gene gdmN of the demycin biosynthetic gene cluster (sequence accession number: AY179507). Southern blot experiments were performed using DIG reagent and a non-radioactive nucleic acid labeling and detection kit (Roche) according to the manufacturer's instructions. A DIG-labeled gdmN DNA fragment wa...
Embodiment 2
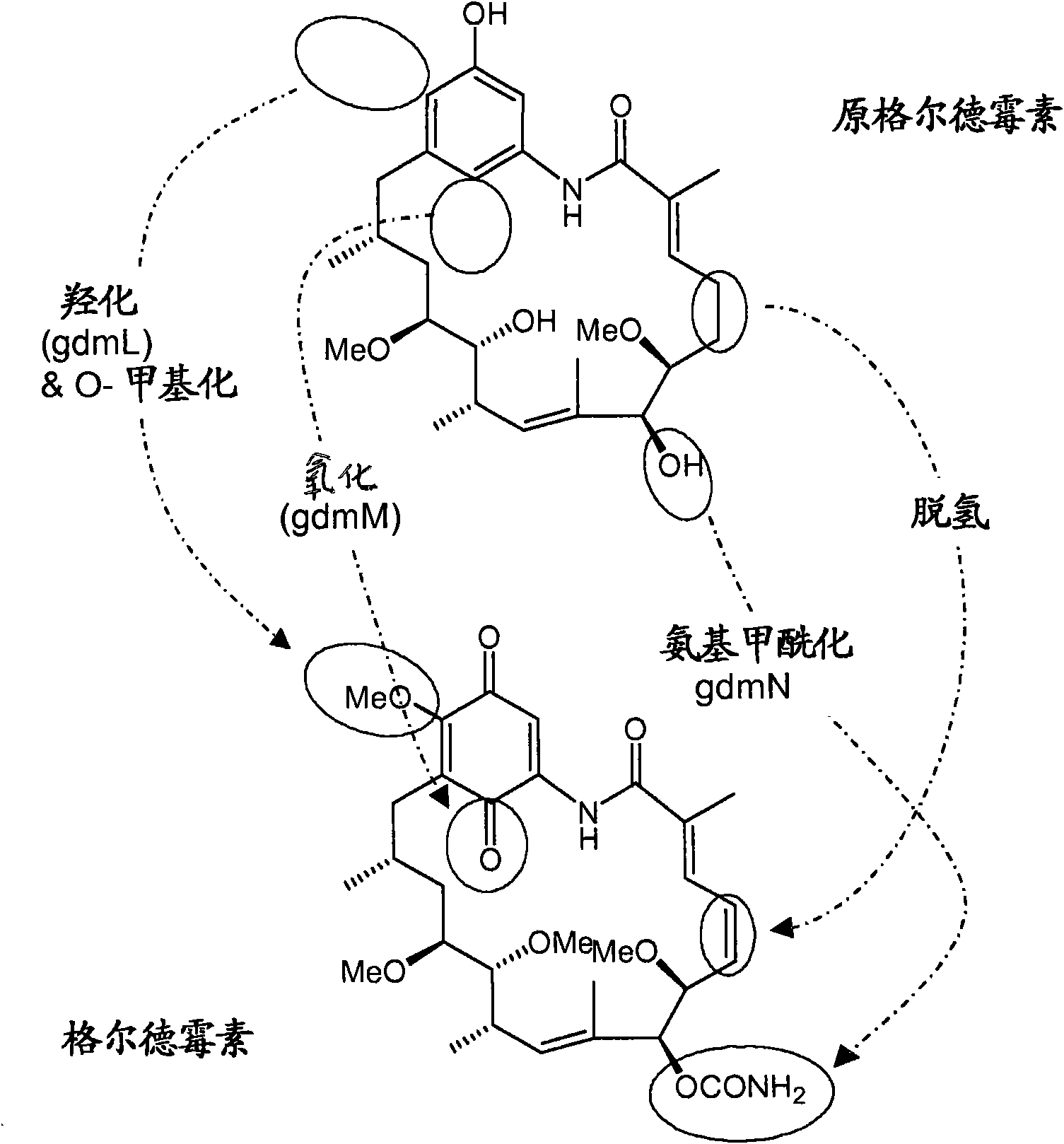
[0355] Example 2 - Generation of gdmM inactivated strains
[0356] In-frame deletion of gdmM was performed as follows:
[0357] 2.1 Cloning of DNA homologous to gdmM upstream flanking regions
[0358] Using oligos gdm1for (SEQ ID NO: 12) and gdm1rev (SEQ ID NO: 13) to amplify Streptomyces hygroscopicus subsp. gordae using genomic DNA as a template in a standard PCR reaction using Pfu DNA polymerase (NRRL 3602) A region of 2268 bp in DNA. A 5' extension in each oligo introduces restriction sites to aid in cloning of the amplified fragments. The PCR product (PCRgdm1 ) covers the region of homology upstream of gdmM up to and including the first two amino acids of gdmM. This 2268bp fragment was cloned into pUC18 linearized with SmaI and dephosphorylated to obtain pUC18gdm1.
[0359] gdm1for TTAAGCTTGGACCGGCGCGAACTCGCGGACACCCACCT
[0360] Hind III
[0361] (SEQ ID NO: 12)
[0362] gdm1revTTTCTAGAGGTCATGCGCCCGCCAGGATCAGGTCGACC
[0363] wxya
[0364] (...
Embodiment 3
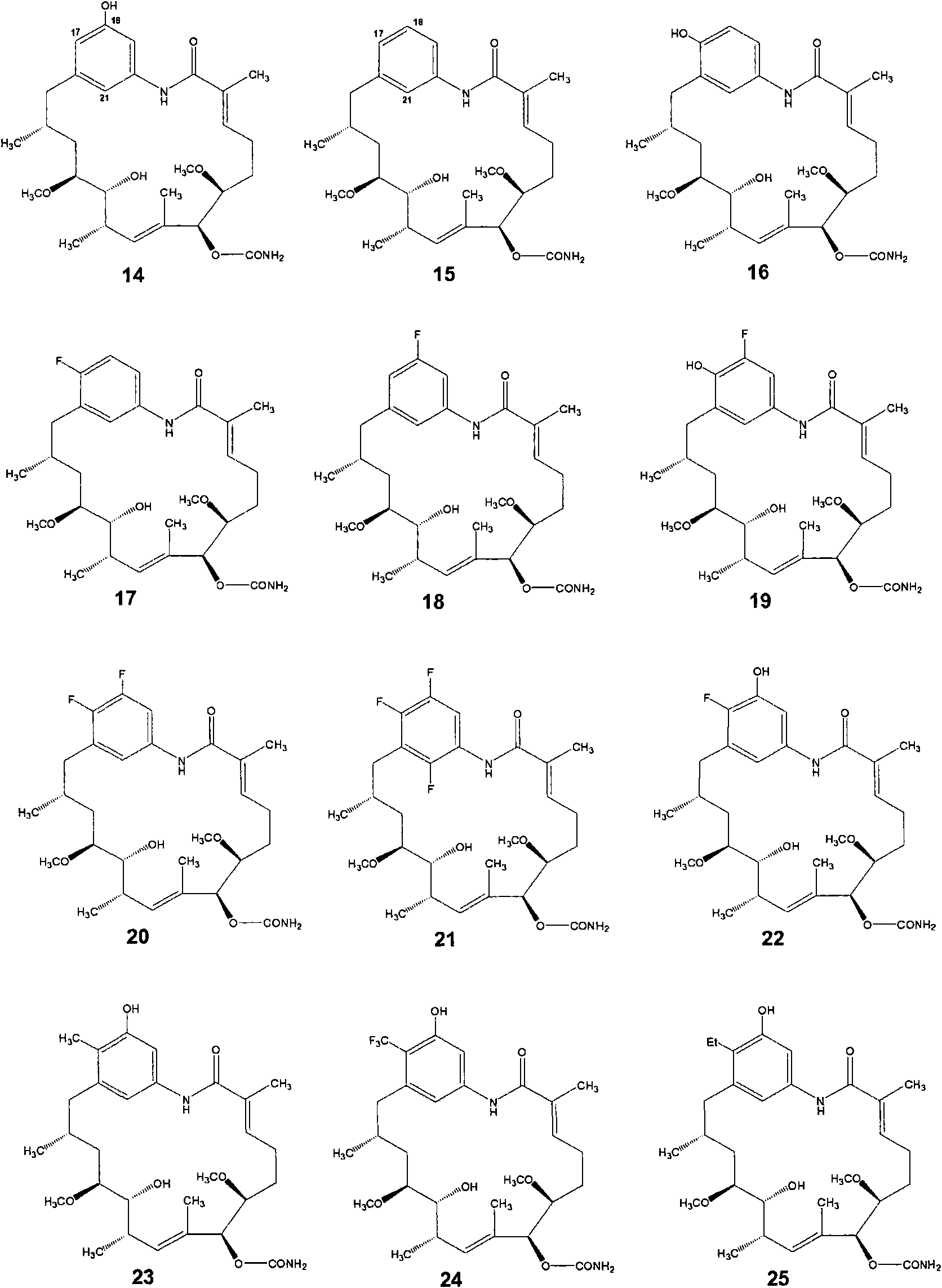
[0383] Example 3 - Strain Streptomyces hygroscopicus gdmM by knockout to gdmM -Supply of AHBA analogs to generate new geldanamycin analogs
[0384] 3.1 Using Streptomyces hygroscopicus gdmM - carry out biotransformation
[0385] Streptomyces hygroscopicus gdmM - As described in Example 2 above, the plaques thereof were transferred to MAM plates (medium 1) and cultured at 28°C for three days. Individual 50 mL falcon tubes were inoculated using 6 mm cylinders, each tube containing 10 mL of seed medium (per liter: glucose 40 g, beet molasses 10 g, yeast extract 2.5 g, peptone 2.5 g, tryptone 2.5 g, oat flakes 5 g) . These seed cultures were incubated at 28°C for 36-72 hours at 300 rpm with a 1 inch eccentricity. These were then used to inoculate (0.5 mL to 10 mL) production medium (same as seed medium) and cultivated at 28°C for 24 hours. 0.1 mL of 200 mM feed stock solution (dissolved in methanol - see Table 4 for list) was added to each falcon tube to give a final fee...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com