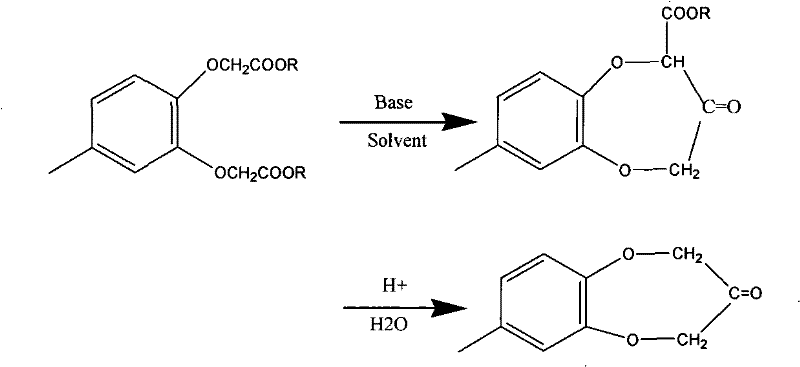
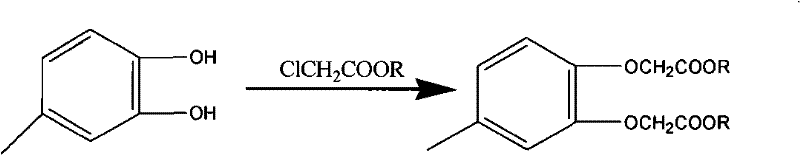Method for synthesizing 4-methyl-1, 2-phenylenedioxyacetic ester
A technology of dioxyacetate and synthesis method, which is applied in the field of synthesis of aromatic ring compounds, can solve the problems of high cost, low reaction yield, unfriendly environment, etc., and achieve low cost, high yield, and easy industrial production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0022] Example 1: Synthesis of methyl 4-methyl-1,2-phenylene dioxyacetate
[0023] In a 1 liter flask, add 57.5g (0.46mol) of 4-methylcatechol, 2.9g of tetrabutylammonium bromide, 419g of butanone, 165g (1.2mol) of anhydrous potassium carbonate and 108g (1mol) of chloroacetic acid The methyl ester was reacted under reflux for 7 hours, filtered after cooling, the filtrate recovered the solvent and then distilled under reduced pressure to collect the 160-162 / 3mmHg fraction to obtain 105 g of the product with a purity of 99% and a yield of 85%.
example 2
[0024] Example 2: Synthesis of methyl 4-methyl-1,2-phenylene dioxyacetate
[0025] In a 1 liter flask, add 57.5g (0.46mol) of 4-methylcatechol, 2.9g of trimethylbenzylammonium chloride, 430g of methyl isobutyl ketone, 138g (1.3mol) of anhydrous sodium carbonate and 162g (1.5mol) methyl chloroacetate was refluxed for 6h, cooled and filtered, the filtrate recovered the solvent and distilled under reduced pressure to collect the 160-162 / 3mmHg fraction to obtain the product 120.8g with a purity of 99% and a yield of 98%.
example 3
[0026] Example 3: Synthesis of methyl 4-methyl-1,2-phenylene dioxyacetate
[0027] In a 1 liter flask, add 57.5g (0.46mol) of 4-methylcatechol, 3.5g of trimethylbenzylammonium chloride, 588g of cyclopentanone, 138g (1.3mol) of anhydrous sodium carbonate and 153g (1.42) mol) methyl chloroacetate, reflux for 6h, cooled and filtered, the filtrate recovered the solvent and distilled under reduced pressure to collect the 160-162 / 3mmHg fraction to obtain the product 113.5g with a purity of 99% and a yield of 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com