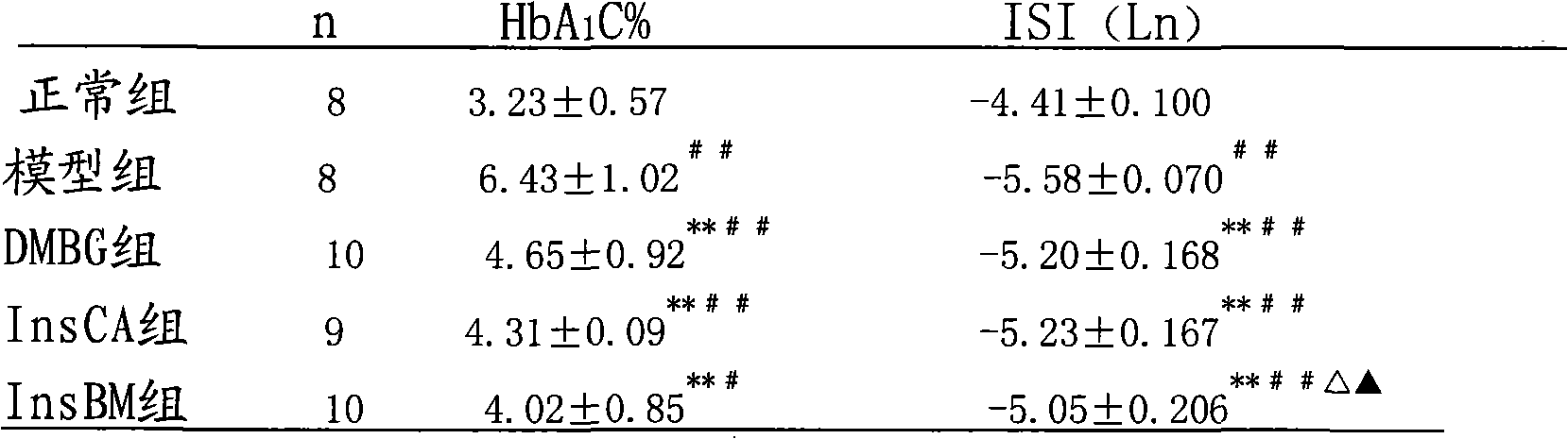Microbiological preparation for preventing or treating diabetes and related symptoms
A technology of microbial preparations and related diseases, which is applied in the field of medicine to achieve the effect of reducing the amount of absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
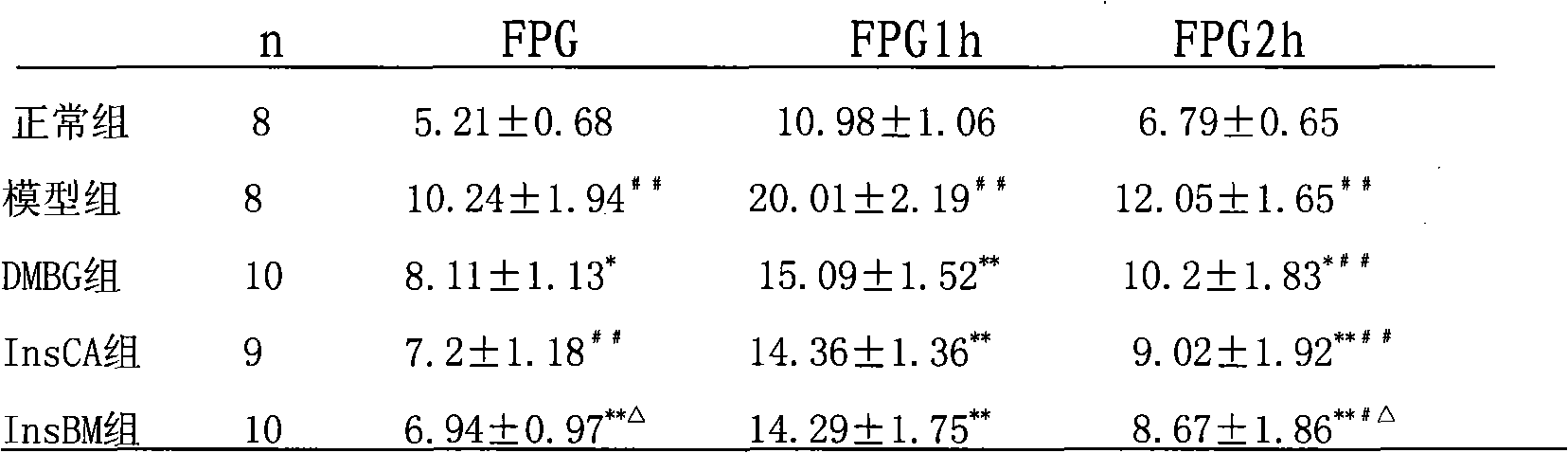
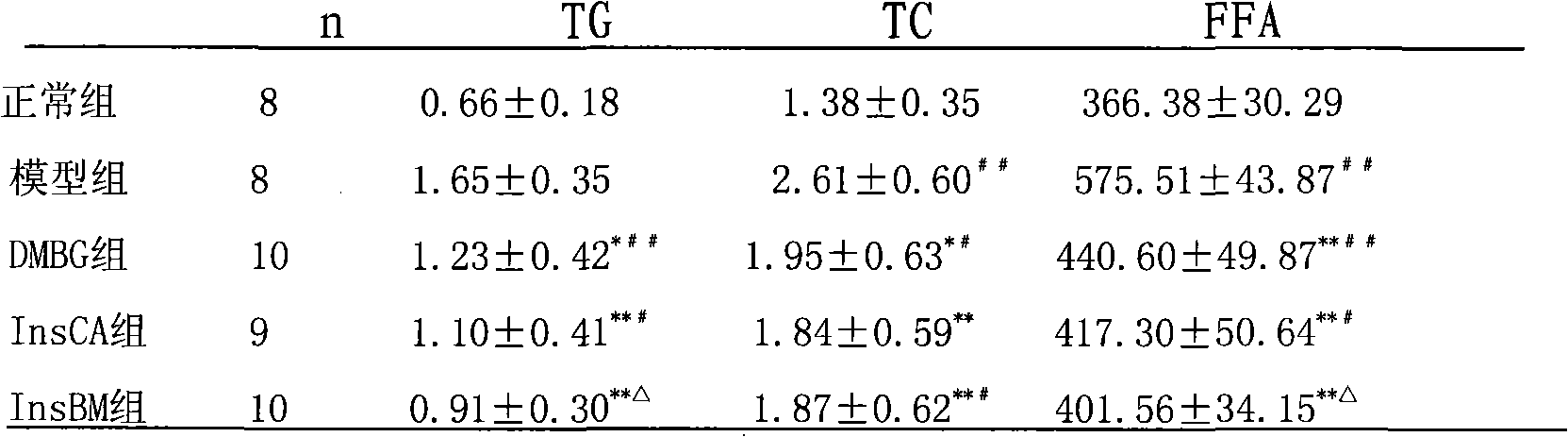
[0071] Example 1 .Effects of InsBM and InsCA on hyperglycemia rats induced by high-fat diet-STZ
[0072] Objective: To study the effects of InsBM and InsCA on the diabetic rat model induced by high-fat diet combined with streptozotocin (STZ). Methods: After feeding Wistar rats with high-fat diet for 4 weeks, fasting for 12 hours, intraperitoneally injecting 1.2% streptozotocin 30 mg / kg, and injecting equal volume of citrate buffer to the blank group. One week later, the rats with successful modeling were randomly divided into model group, metformin group, InsCA group and InsBM group. The treatment group was intragastrically administered InsBM and InsCA, both 20ml / kg.bw, and metformin aqueous solution 500mg / kg.bw; the model group was intragastrically administered equal volume of distilled water, once a day. Blood glucose, plasma insulin, glycosylated hemoglobin and blood lipids were tested 2 weeks after administration, and the insulin sensitivity index was calculated. Resul...
Embodiment 2
[0086] Example 2. Effects of InsBM and InsCA on normal mice and alloxan-induced hyperglycemia mice
[0087] Objective: To study the effects of InsBM and InsCA on blood glucose and glucose tolerance in normal and diabetic mice. Methods: Male ICR mice, weighing 20-24 g. Normal ICR mice were administered orally, and fasting blood glucose and glucose tolerance were measured; alloxan-induced diabetic ICR mouse models were orally administered, and fasting blood glucose and glucose tolerance were measured. Results: InsBM and InsCA had no hypoglycemic effect on normal mice, but they could flatten the glucose tolerance curve; InsBM and InsCA lowered blood glucose in diabetic mice, significantly improved glucose tolerance and resisted the rise of blood glucose in diabetic mice.
[0088] blood sugar Forty normal mice were randomly divided into 4 groups, 10 in each group: the blank control group was gavaged with distilled water, and the positive control group, InsBM group and InsCA...
Embodiment 3
[0107] Example 3. Effects of InsBM and InsCA on Experimental Obese Rats
[0108] Objective: To study the effect of InsBM and InsCA on weight loss in nutritionally obese rats. Methods: Weaned Wistar male rats, weighing 48-72 g, with an average of (55.14±5.07) g, were randomly divided into two groups, one group was fed with normal diet, and the other group was fed with high-fat diet. After 9 weeks, a rat model of nutritional obesity was made. They were randomly divided into 4 groups, namely model group, metformin group, InsCA group and InsBM group. Metformin aqueous solution 500mg / kg.bw, InsBM, InsCA both 20ml / kg.bw were administered intragastrically, and the model group was intragastrically administered an equal volume of distilled water. Each group was administered once a day for 9 consecutive weeks. Body weight, food intake, and testicular fat weight were recorded. Results: After administration, the body weight of rats in InsBM group, InsCA group and metformin group inc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com