Nonylphenol polyoxyethylene ether dimeric surfactant using piperazine as connecting group
A technology of nonylphenol polyoxyethylene ether and surfactant, which is applied in the field of nonylphenol polyoxyethylene ether dimerization surfactant and its preparation, can solve the problems of complex synthesis process, high raw material cost and high reaction temperature, Achieve the effect of avoiding by-products, clear route and high crystallinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Synthesis of Nonylphenol Polyoxyethylene Ether-4 Dimeric Surfactant Bis(NP)-4
[0025] The first step, intermediate N, the synthesis of N'-two (2-hydroxyl-5-nonylphenylmethyl) piperazine:
[0026] (1) Weigh the material according to the molar ratio of anhydrous piperazine:paraformaldehyde=1:2, add it to a three-necked flask, then add dehydrated alcohol 20 times the quality of anhydrous piperazine, turn on the stirring and reflux device, heat and keep The temperature of the material was 50°C, and the reaction was carried out for 2 hours to obtain the intermediate N,N'-dimethylolpiperazine.
[0027] In operation, the amount of paraformaldehyde used above is based on the molar mass of unimolecular formaldehyde (the same as in the following examples).
[0028] (2) Add commercially available industrial product nonylphenol dropwise to the reactant in the three-necked flask of the previous step reaction to continue the reaction. And keep the temperature of the material at 80...
Embodiment 2
[0032] Synthesis of Nonylphenol Polyoxyethylene Ether-30 Dimeric Surfactant Bis(NP)-30
[0033] The first step, intermediate N, the synthesis of N'-two (2-hydroxyl-5-nonylphenylmethyl) piperazine:
[0034](1) Weigh the material according to the molar ratio of anhydrous piperazine:paraformaldehyde=1:4, add it to a three-necked flask, then add dehydrated alcohol 20 times the quality of anhydrous piperazine, open the stirring and reflux device, heat and keep The temperature of the material was 60°C, and the reaction was carried out for 2 hours to obtain the intermediate N,N'-dimethylolpiperazine.
[0035] (2) Add commercially available industrial product nonylphenol dropwise to the reactant in the three-necked flask of the previous step reaction to continue the reaction, the addition of nonylphenol is 3.5 times the molar amount of anhydrous piperazine added in the previous step, heat And keep the temperature of the material at 70°C, react for 5 hours, cool, precipitate white cry...
Embodiment 3~6
[0039] The molar ratio of piperazine to formaldehyde, the molar ratio of nonylphenol to N,N'-dimethylolpiperazine and the number of moles of ethylene oxide added in Examples 3 to 6 are shown in the table below, and other synthetic process steps are implemented in the same way example 1.
[0040]
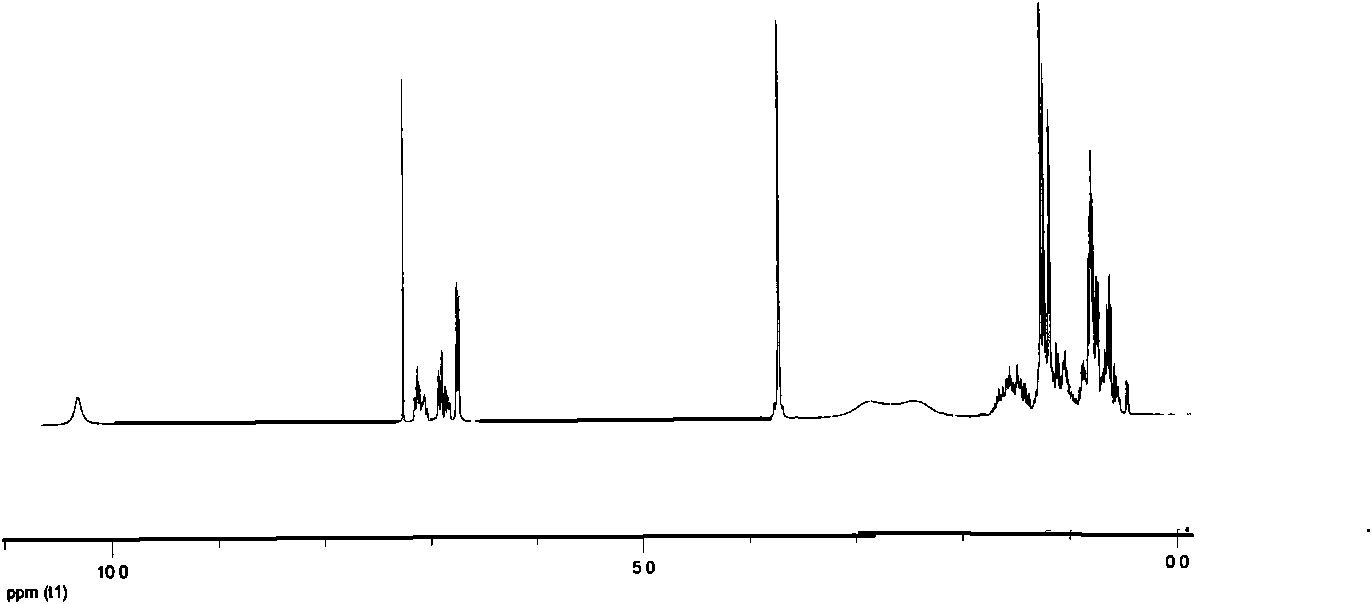
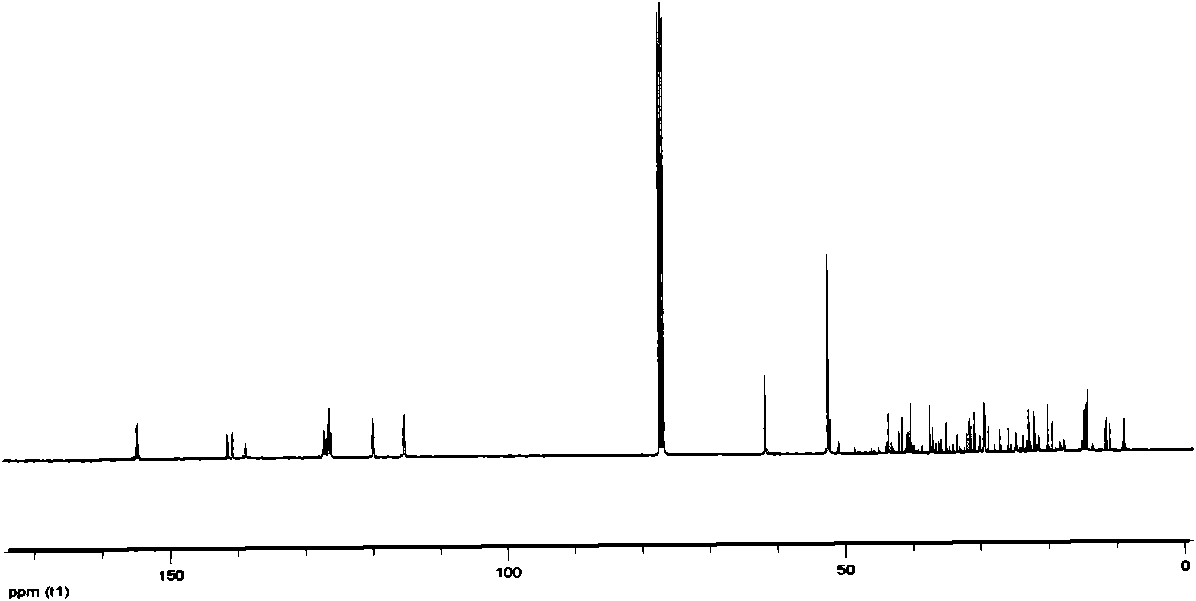
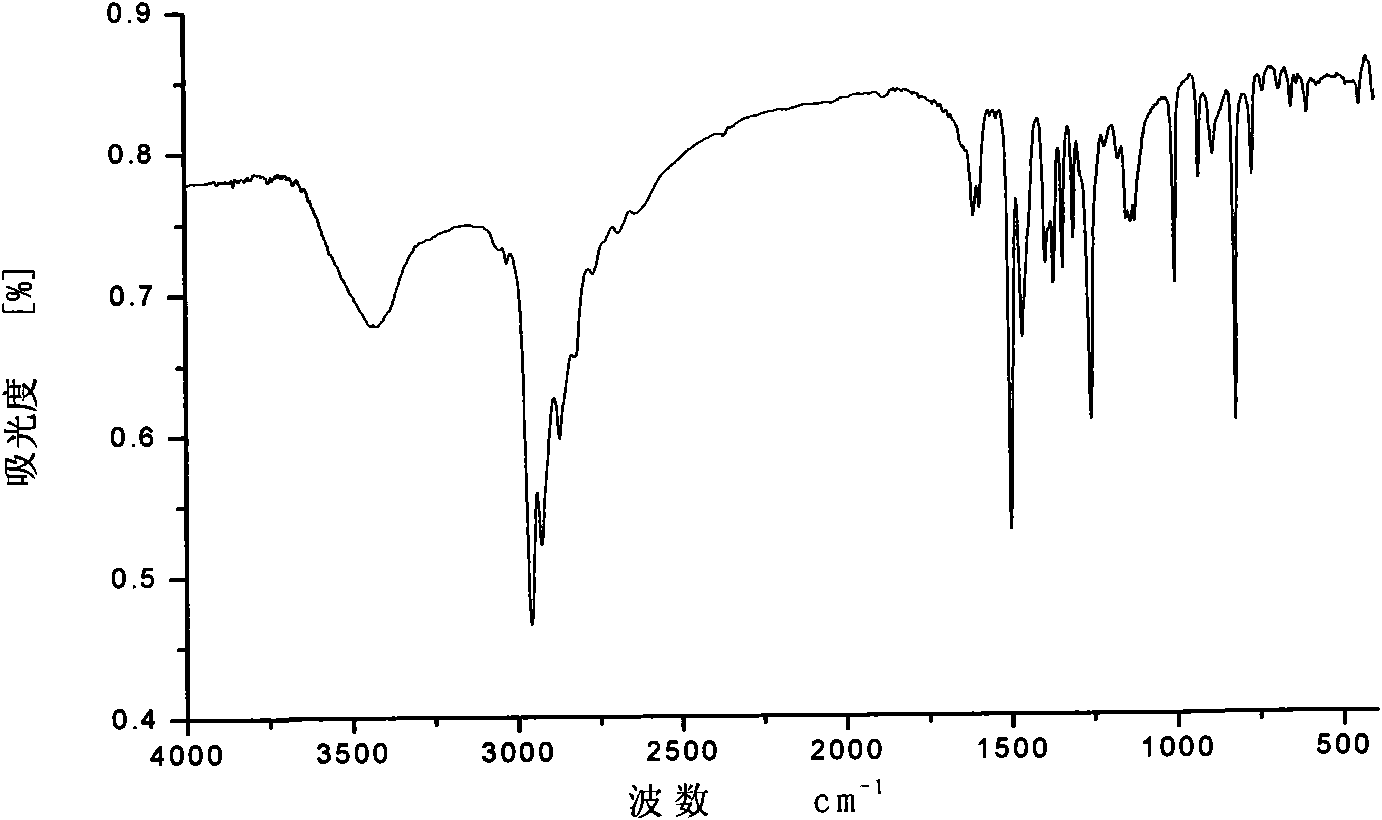
[0041] The above examples illustrate that the present invention adopts the nonylphenol polyoxyethylene ether dimerized surfactant synthesized by the classic Mannich reaction mechanism, and the intermediate is separated and purified by recrystallization, which has the advantages of easy separation and high crystallinity. Since the piperazine as the linking group is a secondary amine, the generation of other by-products can be effectively avoided. The product was characterized by H NMR, C NMR, and IR spectra to accurately analyze the structure of the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com